Biochemical Screening of Phytochemicals and Identification of Scopoletin as a Potential Inhibitor of SARS-CoV-2 Mpro, Revealing Its Biophysical Impact on Structural Stability
et al., Viruses, doi:10.3390/v17030402, Mar 2025
Quercetin for COVID-19
36th treatment shown to reduce risk in
January 2022, now with p = 0.0018 from 9 studies.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
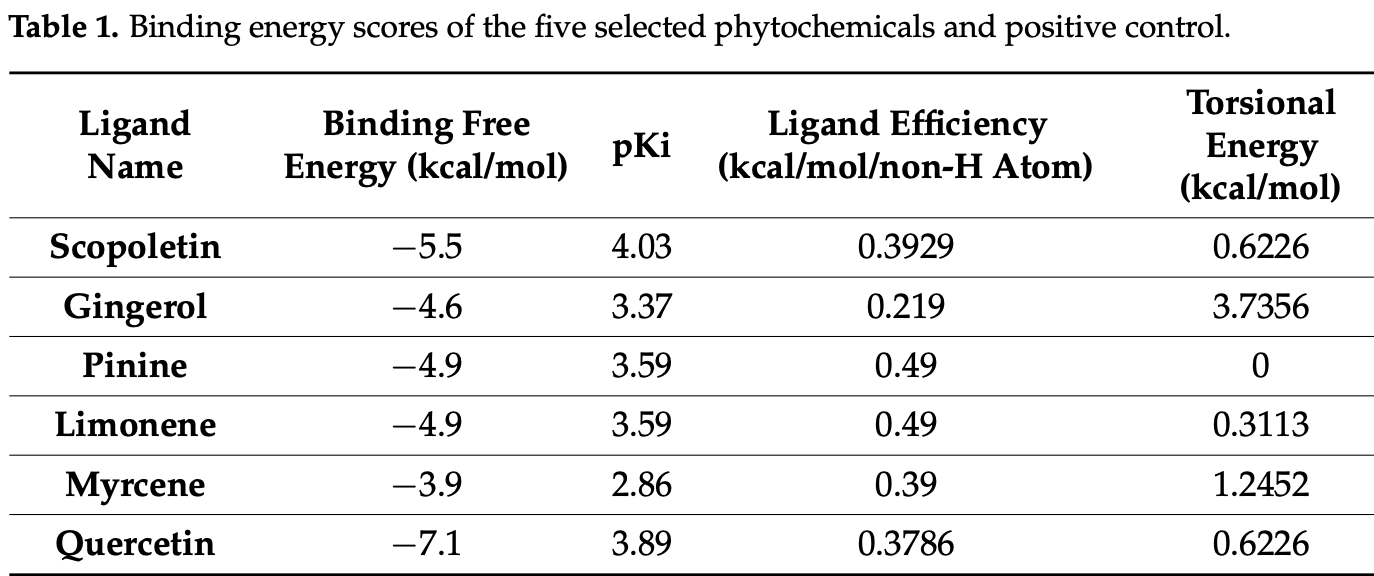
In vitro study showing that scopoletin inhibits SARS-CoV-2 main protease (Mpro) with an IC50 of 15.75 μM. Authors employed virtual screening to identify scopoletin among five phytochemicals as a potential Mpro inhibitor, which was confirmed through FRET-based enzymatic assays. Fluorescence spectroscopy revealed strong binding (KA = 3.17 x 104 M-1) between scopoletin and Mpro, while CD spectroscopy demonstrated significant reduction in helical content upon interaction. Isothermal titration calorimetry supported these findings with a binding constant of 2.36 × 103 M-1. Molecular dynamics simulations over 100 ns confirmed stable complex formation with hydrogen bonding near the active site.
Quercetin was used as a positive control, showing the strongest in silico affinity among screened ligands (binding free energy = -7.1 kcal mol⁻¹, pKi ≈ 3.9) and forming key hydrogen bonds with Tyr54 and Thr26 inside the Mpro active site. In the FRET enzymatic assay, it inhibited Mpro with an IC₅₀ of 49.6 µM, while fluorescence-quenching analysis yielded a Stern-Volmer constant of 1.12 x 10⁶ M⁻¹ and a 1:1 binding constant of 5.34 x 10⁵ M⁻¹. Isothermal-titration calorimetry confirmed tighter binding, giving KA = 7.82 x 10⁴ M⁻¹ (KD = 1.27 x 10⁻⁵ M) and an exothermic ΔH of -615 kJ mol⁻¹, indicating a spontaneous, enthalpy-driven interaction. Cytotoxicity tests in HEK-293 cells showed no appreciable viability loss.
Bioavailability. Quercetin has low bioavailability and studies typically use advanced formulations to improve bioavailability which may be required to reach therapeutic concentrations.
91 preclinical studies support the efficacy of quercetin for COVID-19:
In silico studies predict inhibition of SARS-CoV-2, or minimization of side effects, with quercetin or metabolites via binding to the spikeA,11,12,18,19,32,34,35,37,40,48,49,51,52,75 (and specifically the receptor binding domainB,8), MproC,7,8,11,12,16,18,20,22,24,26,28,30,33,34,37,40,44,46-48,52-55,72 , RNA-dependent RNA polymeraseD,8,10-12,18,42 , PLproE,12,47,55 , ACE2F,27,32,33,37,38,47,51 , TMPRSS2G,32, nucleocapsidH,12, helicaseI,12,39,44 , endoribonucleaseJ,49, NSP16/10K,15, cathepsin LL,36, Wnt-3M,32, FZDN,32, LRP6O,32, ezrinP,50, ADRPQ,48, NRP1R,51, EP300S,25, PTGS2T,33, HSP90AA1U,25,33 , matrix metalloproteinase 9V,41, IL-6W,31,45 , IL-10X,31, VEGFAY,45, and RELAZ,45 proteins, and inhibition of spike-ACE2 interactionAA,9.
In vitro studies demonstrate inhibition of the MproC,24,58,63,71 protein, and inhibition of spike-ACE2 interactionAA,59.
In vitro studies demonstrate efficacy in Calu-3AB,62, A549AC,31, HEK293-ACE2+AD,70, Huh-7AE,35, Caco-2AF,61, Vero E6AG,29,52,61 , mTECAH,64, RAW264.7AI,64, and HLMECAJ,9 cells.
Animal studies demonstrate efficacy in K18-hACE2 miceAK,67, db/db miceAL,64,74 , BALB/c miceAM,73, and rats29.
Quercetin reduced proinflammatory cytokines and protected lung and kidney tissue against LPS-induced damage in mice73, inhibits LPS-induced cytokine storm by modulating key inflammatory and antioxidant pathways in macrophages14, may block ACE2-spike interaction and NLRP3 inflammasome, limiting viral entry and inflammation5, upregulates the SIRT1/AMPK axis to inhibit oxidative injury and accelerate viral clearance76, inhibits SARS-CoV-2 ORF3a ion channel activity, which contributes to viral pathogenicity and cytotoxicity66, may alleviate COVID-19 ARDS via inhibition of EGFR and JAK2 inflammatory targets1, may destabilize the Spike protein, IL-6R, and integrins via conserved residues, blocking viral entry, hyperinflammation, and platelet aggregation77, and may reduce COVID-19 neuroinflammation and cognitive dysfunction through anti-inflammatory mechanisms and neuroprotective effects78.
1.
Gupta et al., Harnessing phytoconstituents to treat COVID-19 triggered acute respiratory distress syndrome: Insights from network pharmacology, and molecular modeling, Phytochemistry Letters, doi:10.1016/j.phytol.2025.104105.
2.
Sun et al., Feasibility of the inhibitor development for SARS-CoV-2: a systematic approach for drug design, Journal of Molecular Modeling, doi:10.1007/s00894-025-06541-2.
3.
Torabfam et al., Improving quercetin solubility via structural modification enhances dual-target coronavirus entry: an integrated in-vitro and in-silico study, Scientific Reports, doi:10.1038/s41598-025-27374-2.
4.
Abdelhameed et al., Phytochemical and antiviral investigation of Cynanchum acutum L. extract and derived semi-synthetic analogs targeting SARS-CoV-2 main protease, Future Journal of Pharmaceutical Sciences, doi:10.1186/s43094-025-00907-2.
5.
Manikyam et al., INP-Guided Network Pharmacology Discloses Multi-Target Therapeutic Strategy Against Cytokine and IgE Storms in the SARS-CoV-2 NB.1.8.1 Variant, Research Square, doi:10.21203/rs.3.rs-6819274/v1.
6.
Makoana et al., Integration of metabolomics and chemometrics with in-silico and in-vitro approaches to unravel SARS-Cov-2 inhibitors from South African plants, PLOS ONE, doi:10.1371/journal.pone.0320415.
7.
Bano et al., Biochemical Screening of Phytochemicals and Identification of Scopoletin as a Potential Inhibitor of SARS-CoV-2 Mpro, Revealing Its Biophysical Impact on Structural Stability, Viruses, doi:10.3390/v17030402.
8.
Rajamanickam et al., Exploring the Potential of Siddha Formulation MilagaiKudineer-Derived Phytotherapeutics Against SARS-CoV-2: An In-Silico Investigation for Antiviral Intervention, Journal of Pharmacy and Pharmacology Research, doi:10.26502/fjppr.0105.
9.
Moharram et al., Secondary metabolites of Alternaria alternate appraisal of their SARS-CoV-2 inhibitory and anti-inflammatory potentials, PLOS ONE, doi:10.1371/journal.pone.0313616.
10.
Metwaly et al., Integrated study of Quercetin as a potent SARS-CoV-2 RdRp inhibitor: Binding interactions, MD simulations, and In vitro assays, PLOS ONE, doi:10.1371/journal.pone.0312866.
11.
Al balawi et al., Assessing multi-target antiviral and antioxidant activities of natural compounds against SARS-CoV-2: an integrated in vitro and in silico study, Bioresources and Bioprocessing, doi:10.1186/s40643-024-00822-z.
12.
Haque et al., Exploring potential therapeutic candidates against COVID-19: a molecular docking study, Discover Molecules, doi:10.1007/s44345-024-00005-5.
13.
Pan et al., Decoding the mechanism of Qingjie formula in the prevention of COVID-19 based on network pharmacology and molecular docking, Heliyon, doi:10.1016/j.heliyon.2024.e39167.
14.
Xu et al., Quercetin inhibited LPS-induced cytokine storm by interacting with the AKT1-FoxO1 and Keap1-Nrf2 signaling pathway in macrophages, Scientific Reports, doi:10.1038/s41598-024-71569-y.
15.
Tamil Selvan et al., Computational Investigations to Identify Potent Natural Flavonoid Inhibitors of the Nonstructural Protein (NSP) 16/10 Complex Against Coronavirus, Cureus, doi:10.7759/cureus.68098.
16.
Sunita et al., Characterization of Phytochemical Inhibitors of the COVID-19 Primary Protease Using Molecular Modelling Approach, Asian Journal of Microbiology and Biotechnology, doi:10.56557/ajmab/2024/v9i28800.
17.
Wu et al., Biomarkers Prediction and Immune Landscape in Covid-19 and “Brain Fog”, Elsevier BV, doi:10.2139/ssrn.4897774.
18.
Raman et al., Phytoconstituents of Citrus limon (Lemon) as Potential Inhibitors Against Multi Targets of SARS‐CoV‐2 by Use of Molecular Modelling and In Vitro Determination Approaches, ChemistryOpen, doi:10.1002/open.202300198.
19.
Asad et al., Exploring the antiviral activity of Adhatoda beddomei bioactive compounds in interaction with coronavirus spike protein, Archives of Medical Reports, 1:1, archmedrep.com/index.php/amr/article/view/3.
20.
Irfan et al., Phytoconstituents of Artemisia Annua as potential inhibitors of SARS CoV2 main protease: an in silico study, BMC Infectious Diseases, doi:10.1186/s12879-024-09387-w.
21.
Yuan et al., Network pharmacology and molecular docking reveal the mechanisms of action of Panax notoginseng against post-COVID-19 thromboembolism, Review of Clinical Pharmacology and Pharmacokinetics - International Edition, doi:10.61873/DTFA3974.
22.
Nalban et al., Targeting COVID-19 (SARS-CoV-2) main protease through phytochemicals of Albizia lebbeck: molecular docking, molecular dynamics simulation, MM–PBSA free energy calculations, and DFT analysis, Journal of Proteins and Proteomics, doi:10.1007/s42485-024-00136-w.
23.
Zhou et al., Bioinformatics and system biology approaches to determine the connection of SARS-CoV-2 infection and intrahepatic cholangiocarcinoma, PLOS ONE, doi:10.1371/journal.pone.0300441.
24.
Waqas et al., Discovery of Novel Natural Inhibitors Against SARS-CoV-2 Main Protease: A Rational Approach to Antiviral Therapeutics, Current Medicinal Chemistry, doi:10.2174/0109298673292839240329081008.
25.
Hasanah et al., Decoding the therapeutic potential of empon-empon: a bioinformatics expedition unraveling mechanisms against COVID-19 and atherosclerosis, International Journal of Applied Pharmaceutics, doi:10.22159/ijap.2024v16i2.50128.
26.
Shaik et al., Computational identification of selected bioactive compounds from Cedrus deodara as inhibitors against SARS-CoV-2 main protease: a pharmacoinformatics study, Indian Drugs, doi:10.53879/id.61.02.13859.
27.
Wang et al., Investigating the Mechanism of Qu Du Qiang Fei 1 Hao Fang Formula against Coronavirus Disease 2019 Based on Network Pharmacology Method, World Journal of Traditional Chinese Medicine, doi:10.4103/2311-8571.395061.
28.
Singh et al., Unlocking the potential of phytochemicals in inhibiting SARS-CoV-2 M Pro protein - An in-silico and cell-based approach, Research Square, doi:10.21203/rs.3.rs-3888947/v1.
29.
El-Megharbel et al., Chemical and spectroscopic characterization of (Artemisinin/Quercetin/ Zinc) novel mixed ligand complex with assessment of its potent high antiviral activity against SARS-CoV-2 and antioxidant capacity against toxicity induced by acrylamide in male rats, PeerJ, doi:10.7717/peerj.15638.
30.
Akinwumi et al., Evaluation of therapeutic potentials of some bioactive compounds in selected African plants targeting main protease (Mpro) in SARS-CoV-2: a molecular docking study, Egyptian Journal of Medical Human Genetics, doi:10.1186/s43042-023-00456-4.
31.
Yang et al., Active ingredient and mechanistic analysis of traditional Chinese medicine formulas for the prevention and treatment of COVID-19: Insights from bioinformatics and in vitro experiments, Medicine, doi:10.1097/MD.0000000000036238.
32.
Chandran et al., Molecular docking analysis of quercetin with known CoVid-19 targets, Bioinformation, doi:10.6026/973206300191081.
33.
Qin et al., Exploring the bioactive compounds of Feiduqing formula for the prevention and management of COVID-19 through network pharmacology and molecular docking, Medical Data Mining, doi:10.53388/MDM202407003.
34.
Moschovou et al., Exploring the Binding Effects of Natural Products and Antihypertensive Drugs on SARS-CoV-2: An In Silico Investigation of Main Protease and Spike Protein, International Journal of Molecular Sciences, doi:10.3390/ijms242115894.
35.
Pan (B) et al., Quercetin: A promising drug candidate against the potential SARS-CoV-2-Spike mutants with high viral infectivity, Computational and Structural Biotechnology Journal, doi:10.1016/j.csbj.2023.10.029.
36.
Ahmed et al., Evaluation of the Effect of Zinc, Quercetin, Bromelain and Vitamin C on COVID-19 Patients, International Journal of Diabetes Management, doi:10.61797/ijdm.v2i2.259.
37.
Thapa et al., In-silico Approach for Predicting the Inhibitory Effect of Home Remedies on Severe Acute Respiratory Syndrome Coronavirus-2, Makara Journal of Science, doi:10.7454/mss.v27i3.1609.
38.
Alkafaas et al., A study on the effect of natural products against the transmission of B.1.1.529 Omicron, Virology Journal, doi:10.1186/s12985-023-02160-6.
39.
Singh (B) et al., Flavonoids as Potent Inhibitor of SARS-CoV-2 Nsp13 Helicase: Grid Based Docking Approach, Middle East Research Journal of Pharmaceutical Sciences, doi:10.36348/merjps.2023.v03i04.001.
40.
Mandal et al., In silico anti-viral assessment of phytoconstituents in a traditional (Siddha Medicine) polyherbal formulation – Targeting Mpro and pan-coronavirus post-fusion Spike protein, Journal of Traditional and Complementary Medicine, doi:10.1016/j.jtcme.2023.07.004.
41.
Sai Ramesh et al., Computational analysis of the phytocompounds of Mimusops elengi against spike protein of SARS CoV2 – An Insilico model, International Journal of Biological Macromolecules, doi:10.1016/j.ijbiomac.2023.125553.
42.
Corbo et al., Inhibitory potential of phytochemicals on five SARS-CoV-2 proteins: in silico evaluation of endemic plants of Bosnia and Herzegovina, Biotechnology & Biotechnological Equipment, doi:10.1080/13102818.2023.2222196.
43.
Azmi et al., Utilization of quercetin flavonoid compounds in onion (Allium cepa L.) as an inhibitor of SARS-CoV-2 spike protein against ACE2 receptors, 11th International Seminar on New Paradigm and Innovation on Natural Sciences and its Application, doi:10.1063/5.0140285.
44.
Alanzi et al., Structure-based virtual identification of natural inhibitors of SARS-CoV-2 and its Delta and Omicron variant proteins, Future Virology, doi:10.2217/fvl-2022-0184.
45.
Yang (B) et al., In silico evidence implicating novel mechanisms of Prunella vulgaris L. as a potential botanical drug against COVID-19-associated acute kidney injury, Frontiers in Pharmacology, doi:10.3389/fphar.2023.1188086.
46.
Wang (B) et al., Computational Analysis of Lianhua Qingwen as an Adjuvant Treatment in Patients with COVID-19, Society of Toxicology Conference, 2023, www.researchgate.net/publication/370491709_Y_Wang_A_E_Tan_O_Chew_A_Hsueh_and_D_E_Johnson_2023_Computational_Analysis_of_Lianhua_Qingwen_as_an_Adjuvant_Treatment_in_Patients_with_COVID-19_Toxicologist_1921_507.
47.
Ibeh et al., Computational studies of potential antiviral compounds from some selected Nigerian medicinal plants against SARS-CoV-2 proteins, Informatics in Medicine Unlocked, doi:10.1016/j.imu.2023.101230.
48.
Nguyen et al., The Potential of Ameliorating COVID-19 and Sequelae From Andrographis paniculata via Bioinformatics, Bioinformatics and Biology Insights, doi:10.1177/11779322221149622.
49.
Alavi et al., Interaction of Epigallocatechin Gallate and Quercetin with Spike Glycoprotein (S-Glycoprotein) of SARS-CoV-2: In Silico Study, Biomedicines, doi:10.3390/biomedicines10123074.
50.
Chellasamy et al., Docking and molecular dynamics studies of human ezrin protein with a modelled SARS-CoV-2 endodomain and their interaction with potential invasion inhibitors, Journal of King Saud University - Science, doi:10.1016/j.jksus.2022.102277.
51.
Şimşek et al., In silico identification of SARS-CoV-2 cell entry inhibitors from selected natural antivirals, Journal of Molecular Graphics and Modelling, doi:10.1016/j.jmgm.2021.108038.
52.
Kandeil et al., Bioactive Polyphenolic Compounds Showing Strong Antiviral Activities against Severe Acute Respiratory Syndrome Coronavirus 2, Pathogens, doi:10.3390/pathogens10060758.
53.
Rehman et al., Natural Compounds as Inhibitors of SARS-CoV-2 Main Protease (3CLpro): A Molecular Docking and Simulation Approach to Combat COVID-19, Current Pharmaceutical Design, doi:10.2174/1381612826999201116195851.
54.
Sekiou et al., In-Silico Identification of Potent Inhibitors of COVID-19 Main Protease (Mpro) and Angiotensin Converting Enzyme 2 (ACE2) from Natural Products: Quercetin, Hispidulin, and Cirsimaritin Exhibited Better Potential Inhibition than Hydroxy-Chloroquine Against COVID-19 Main Protease Active Site and ACE2, ChemRxiv, doi:10.26434/chemrxiv.12181404.v1.
55.
Zhang et al., In silico screening of Chinese herbal medicines with the potential to directly inhibit 2019 novel coronavirus, Journal of Integrative Medicine, doi:10.1016/j.joim.2020.02.005.
56.
Sisti et al., Evaluation of respiratory virus transmissibility and resilience from fomites: the case of 11 SARS-CoV-2 clinical isolates, Applied and Environmental Microbiology, doi:10.1128/aem.00774-25.
57.
Spinelli et al., Amphibian‐Derived Peptides as Natural Inhibitors of SARS‐CoV‐2 Main Protease (Mpro): A Combined In Vitro and In Silico Approach, Chemistry & Biodiversity, doi:10.1002/cbdv.202403202.
58.
Aguilera-Rodriguez et al., Inhibition of SARS-CoV-2 3CLpro by chemically modified tyrosinase from Agaricus bisporus, RSC Medicinal Chemistry, doi:10.1039/D4MD00289J.
59.
Emam et al., Establishment of in-house assay for screening of anti-SARS-CoV-2 protein inhibitors, AMB Express, doi:10.1186/s13568-024-01739-8.
60.
Fang et al., Development of nanoparticles incorporated with quercetin and ACE2-membrane as a novel therapy for COVID-19, Journal of Nanobiotechnology, doi:10.1186/s12951-024-02435-2.
61.
Roy et al., Quercetin inhibits SARS-CoV-2 infection and prevents syncytium formation by cells co-expressing the viral spike protein and human ACE2, Virology Journal, doi:10.1186/s12985-024-02299-w.
62.
DiGuilio et al., Quercetin improves and protects Calu-3 airway epithelial barrier function, Frontiers in Cell and Developmental Biology, doi:10.3389/fcell.2023.1271201.
63.
Zhang (B) et al., Discovery of the covalent SARS‐CoV‐2 Mpro inhibitors from antiviral herbs via integrating target‐based high‐throughput screening and chemoproteomic approaches, Journal of Medical Virology, doi:10.1002/jmv.29208.
64.
Wu (B) et al., SARS-CoV-2 N protein induced acute kidney injury in diabetic db/db mice is associated with a Mincle-dependent M1 macrophage activation, Frontiers in Immunology, doi:10.3389/fimmu.2023.1264447.
65.
Xu (B) et al., Bioactive compounds from Huashi Baidu decoction possess both antiviral and anti-inflammatory effects against COVID-19, Proceedings of the National Academy of Sciences, doi:10.1073/pnas.2301775120.
66.
Fam et al., Channel activity of SARS-CoV-2 viroporin ORF3a inhibited by adamantanes and phenolic plant metabolites, Scientific Reports, doi:10.1038/s41598-023-31764-9.
67.
Aguado et al., Senolytic therapy alleviates physiological human brain aging and COVID-19 neuropathology, bioRxiv, doi:10.1101/2023.01.17.524329.
68.
Goc et al., Inhibitory effects of specific combination of natural compounds against SARS-CoV-2 and its Alpha, Beta, Gamma, Delta, Kappa, and Mu variants, European Journal of Microbiology and Immunology, doi:10.1556/1886.2021.00022.
69.
Munafò et al., Quercetin and Luteolin Are Single-digit Micromolar Inhibitors of the SARS-CoV-2 RNA-dependent RNA Polymerase, Research Square, doi:10.21203/rs.3.rs-1149846/v1.
70.
Singh (C) et al., The spike protein of SARS-CoV-2 virus induces heme oxygenase-1: Pathophysiologic implications, Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease, doi:10.1016/j.bbadis.2021.166322.
71.
Bahun et al., Inhibition of the SARS-CoV-2 3CLpro main protease by plant polyphenols, Food Chemistry, doi:10.1016/j.foodchem.2021.131594.
72.
Abian et al., Structural stability of SARS-CoV-2 3CLpro and identification of quercetin as an inhibitor by experimental screening, International Journal of Biological Macromolecules, doi:10.1016/j.ijbiomac.2020.07.235.
73.
Shaker et al., Anti-cytokine Storm Activity of Fraxin, Quercetin, and their Combination on Lipopolysaccharide-Induced Cytokine Storm in Mice: Implications in COVID-19, Iranian Journal of Medical Sciences, doi:10.30476/ijms.2023.98947.3102.
74.
Wu (C) et al., Treatment with Quercetin inhibits SARS-CoV-2 N protein-induced acute kidney injury by blocking Smad3-dependent G1 cell cycle arrest, Molecular Therapy, doi:10.1016/j.ymthe.2022.12.002.
75.
Azmi (B) et al., The role of vitamin D receptor and IL‐6 in COVID‐19, Molecular Genetics & Genomic Medicine, doi:10.1002/mgg3.2172.
76.
Shokri-Afra et al., Targeting SIRT1: A Potential Strategy for Combating Severe COVID‐19, BioMed Research International, doi:10.1155/bmri/9507417.
a.
The trimeric spike (S) protein is a glycoprotein that mediates viral entry by binding to the host ACE2 receptor, is critical for SARS-CoV-2's ability to infect host cells, and is a target of neutralizing antibodies. Inhibition of the spike protein prevents viral attachment, halting infection at the earliest stage.
b.
The receptor binding domain is a specific region of the spike protein that binds ACE2 and is a major target of neutralizing antibodies. Focusing on the precise binding site allows highly specific disruption of viral attachment with reduced potential for off-target effects.
c.
The main protease or Mpro, also known as 3CLpro or nsp5, is a cysteine protease that cleaves viral polyproteins into functional units needed for replication. Inhibiting Mpro disrupts the SARS-CoV-2 lifecycle within the host cell, preventing the creation of new copies.
d.
RNA-dependent RNA polymerase (RdRp), also called nsp12, is the core enzyme of the viral replicase-transcriptase complex that copies the positive-sense viral RNA genome into negative-sense templates for progeny RNA synthesis. Inhibiting RdRp blocks viral genome replication and transcription.
e.
The papain-like protease (PLpro) has multiple functions including cleaving viral polyproteins and suppressing the host immune response by deubiquitination and deISGylation of host proteins. Inhibiting PLpro may block viral replication and help restore normal immune responses.
f.
The angiotensin converting enzyme 2 (ACE2) protein is a host cell transmembrane protein that serves as the cellular receptor for the SARS-CoV-2 spike protein. ACE2 is expressed on many cell types, including epithelial cells in the lungs, and allows the virus to enter and infect host cells. Inhibition may affect ACE2's physiological function in blood pressure control.
g.
Transmembrane protease serine 2 (TMPRSS2) is a host cell protease that primes the spike protein, facilitating cellular entry. TMPRSS2 activity helps enable cleavage of the spike protein required for membrane fusion and virus entry. Inhibition may especially protect respiratory epithelial cells, buy may have physiological effects.
h.
The nucleocapsid (N) protein binds and encapsulates the viral genome by coating the viral RNA. N enables formation and release of infectious virions and plays additional roles in viral replication and pathogenesis. N is also an immunodominant antigen used in diagnostic assays.
i.
The helicase, or nsp13, protein unwinds the double-stranded viral RNA, a crucial step in replication and transcription. Inhibition may prevent viral genome replication and the creation of new virus components.
j.
The endoribonuclease, also known as NendoU or nsp15, cleaves specific sequences in viral RNA which may help the virus evade detection by the host immune system. Inhibition may hinder the virus's ability to mask itself from the immune system, facilitating a stronger immune response.
k.
The NSP16/10 complex consists of non-structural proteins 16 and 10, forming a 2'-O-methyltransferase that modifies the viral RNA cap structure. This modification helps the virus evade host immune detection by mimicking host mRNA, making NSP16/10 a promising antiviral target.
l.
Cathepsin L is a host lysosomal cysteine protease that can prime the spike protein through an alternative pathway when TMPRSS2 is unavailable. Dual targeting of cathepsin L and TMPRSS2 may maximize disruption of alternative pathways for virus entry.
m.
Wingless-related integration site (Wnt) ligand 3 is a host signaling molecule that activates the Wnt signaling pathway, which is important in development, cell growth, and tissue repair. Some studies suggest that SARS-CoV-2 infection may interfere with the Wnt signaling pathway, and that Wnt3a is involved in SARS-CoV-2 entry.
n.
The frizzled (FZD) receptor is a host transmembrane receptor that binds Wnt ligands, initiating the Wnt signaling cascade. FZD serves as a co-receptor, along with ACE2, in some proposed mechanisms of SARS-CoV-2 infection. The virus may take advantage of this pathway as an alternative entry route.
o.
Low-density lipoprotein receptor-related protein 6 is a cell surface co-receptor essential for Wnt signaling. LRP6 acts in tandem with FZD for signal transduction and has been discussed as a potential co-receptor for SARS-CoV-2 entry.
p.
The ezrin protein links the cell membrane to the cytoskeleton (the cell's internal support structure) and plays a role in cell shape, movement, adhesion, and signaling. Drugs that occupy the same spot on ezrin where the viral spike protein would bind may hindering viral attachment, and drug binding could further stabilize ezrin, strengthening its potential natural capacity to impede viral fusion and entry.
q.
The Adipocyte Differentiation-Related Protein (ADRP, also known as Perilipin 2 or PLIN2) is a lipid droplet protein regulating the storage and breakdown of fats in cells. SARS-CoV-2 may hijack the lipid handling machinery of host cells and ADRP may play a role in this process. Disrupting ADRP's interaction with the virus may hinder the virus's ability to use lipids for replication and assembly.
r.
Neuropilin-1 (NRP1) is a cell surface receptor with roles in blood vessel development, nerve cell guidance, and immune responses. NRP1 may function as a co-receptor for SARS-CoV-2, facilitating viral entry into cells. Blocking NRP1 may disrupt an alternative route of viral entry.
s.
EP300 (E1A Binding Protein P300) is a transcriptional coactivator involved in several cellular processes, including growth, differentiation, and apoptosis, through its acetyltransferase activity that modifies histones and non-histone proteins. EP300 facilitates viral entry into cells and upregulates inflammatory cytokine production.
t.
Prostaglandin G/H synthase 2 (PTGS2, also known as COX-2) is an enzyme crucial for the production of inflammatory molecules called prostaglandins. PTGS2 plays a role in the inflammatory response that can become severe in COVID-19 and inhibitors (like some NSAIDs) may have benefits in dampening harmful inflammation, but note that prostaglandins have diverse physiological functions.
u.
Heat Shock Protein 90 Alpha Family Class A Member 1 (HSP90AA1) is a chaperone protein that helps other proteins fold correctly and maintains their stability. HSP90AA1 plays roles in cell signaling, survival, and immune responses. HSP90AA1 may interact with numerous viral proteins, but note that it has diverse physiological functions.
v.
Matrix metalloproteinase 9 (MMP9), also called gelatinase B, is a zinc-dependent enzyme that breaks down collagen and other components of the extracellular matrix. MMP9 levels increase in severe COVID-19. Overactive MMP9 can damage lung tissue and worsen inflammation. Inhibition of MMP9 may prevent excessive tissue damage and help regulate the inflammatory response.
w.
The interleukin-6 (IL-6) pro-inflammatory cytokine (signaling molecule) has a complex role in the immune response and may trigger and perpetuate inflammation. Elevated IL-6 levels are associated with severe COVID-19 cases and cytokine storm. Anti-IL-6 therapies may be beneficial in reducing excessive inflammation in severe COVID-19 cases.
x.
The interleukin-10 (IL-10) anti-inflammatory cytokine helps regulate and dampen immune responses, preventing excessive inflammation. IL-10 levels can also be elevated in severe COVID-19. IL-10 could either help control harmful inflammation or potentially contribute to immune suppression.
y.
Vascular Endothelial Growth Factor A (VEGFA) promotes the growth of new blood vessels (angiogenesis) and has roles in inflammation and immune responses. VEGFA may contribute to blood vessel leakiness and excessive inflammation associated with severe COVID-19.
z.
RELA is a transcription factor subunit of NF-kB and is a key regulator of inflammation, driving pro-inflammatory gene expression. SARS-CoV-2 may hijack and modulate NF-kB pathways.
aa.
The interaction between the SARS-CoV-2 spike protein and the human ACE2 receptor is a primary method of viral entry, inhibiting this interaction can prevent the virus from attaching to and entering host cells, halting infection at an early stage.
ab.
Calu-3 is a human lung adenocarcinoma cell line with moderate ACE2 and TMPRSS2 expression and SARS-CoV-2 susceptibility. It provides a model of the human respiratory epithelium, but many not be ideal for modeling early stages of infection due to the moderate expression levels of ACE2 and TMPRSS2.
ac.
A549 is a human lung carcinoma cell line with low ACE2 expression and SARS-CoV-2 susceptibility. Viral entry/replication can be studied but the cells may not replicate all aspects of lung infection.
ad.
HEK293-ACE2+ is a human embryonic kidney cell line engineered for high ACE2 expression and SARS-CoV-2 susceptibility.
ae.
Huh-7 cells were derived from a liver tumor (hepatoma).
af.
Caco-2 cells come from a colorectal adenocarcinoma (cancer). They are valued for their ability to form a polarized cell layer with properties similar to the intestinal lining.
ag.
Vero E6 is an African green monkey kidney cell line with low/no ACE2 expression and high SARS-CoV-2 susceptibility. The cell line is easy to maintain and supports robust viral replication, however the monkey origin may not accurately represent human responses.
ah.
mTEC is a mouse tubular epithelial cell line.
ai.
RAW264.7 is a mouse macrophage cell line.
aj.
HLMEC (Human Lung Microvascular Endothelial Cells) are primary endothelial cells derived from the lung microvasculature. They are used to study endothelial function, inflammation, and viral interactions, particularly in the context of lung infections such as SARS-CoV-2. HLMEC express ACE2 and are susceptible to SARS-CoV-2 infection, making them a relevant model for studying viral entry and endothelial responses in the lung.
ak.
A mouse model expressing the human ACE2 receptor under the control of the K18 promoter.
al.
A mouse model of obesity and severe insulin resistance leading to type 2 diabetes due to a mutation in the leptin receptor gene that impairs satiety signaling.
am.
A mouse model commonly used in infectious disease and cancer research due to higher immune response and susceptibility to infection.
Bano et al., 12 Mar 2025, peer-reviewed, 9 authors.
Contact: sdey@acbr.du.ac.in (corresponding author), sbano@acbr.du.ac.in, jyotishnasingh08@gmail.com, zainyzehrajmi@gmail.com, md186547@st.jmi.ac.in, taj144796@st.jmi.ac.in, mihassan@jmi.ac.in, aislam@jmi.ac.in, seemasundari@gmail.com.
In vitro studies are an important part of preclinical research, however results may be very different in vivo.
Biochemical Screening of Phytochemicals and Identification of Scopoletin as a Potential Inhibitor of SARS-CoV-2 Mpro, Revealing Its Biophysical Impact on Structural Stability
Viruses, doi:10.3390/v17030402
The main protease (M pro or 3CL pro or nsp5) of SARS-CoV-2 is crucial to the life cycle and pathogenesis of SARS-CoV-2, making it an attractive drug target to develop antivirals. This study employed the virtual screening of a few phytochemicals, and the resultant best compound, Scopoletin, was further investigated by a FRET-based enzymatic assay, revealing an experimental IC 50 of 15.75 µM. The impact of Scopoletin on M pro was further investigated by biophysical and MD simulation studies. Fluorescence spectroscopy identified a strong binding constant of 3.17 × 10 4 M -1 for Scopoletin binding to M pro , as demonstrated by its effective fluorescence quenching of M pro . Additionally, CD spectroscopy showed a significant reduction in the helical content of M pro upon interaction with Scopoletin. The findings of thermodynamic measurements using isothermal titration calorimetry (ITC) supported the spectroscopic data, indicating a tight binding of Scopoletin to M pro with a K A of 2.36 × 10 3 M -1 . Similarly, interaction studies have also revealed that Scopoletin forms hydrogen bonds with the amino acids nearest to the active site, and this has been further supported by molecular dynamics simulation studies. These findings indicate that Scopoletin may be developed as a potential antiviral treatment for SARS-CoV-2 by targeting M pro .
Supplementary Materials: The following supporting information can be downloaded at: www.mdpi.com/xxx/s1, Figure S1 : UV-vis absorption spectrum of SARS-CoV-2 M pro . Figure S2 : Fluorescence emission spectrum of SARS-CoV-2 M pro , recorded at an excitation of 292 nm.
Supplementary Materials: The following supporting information can be downloaded at: https: //www.mdpi.com/article/10.3390/v17030402/s1 , Figure S1 : UV-vis absorption spectrum of SARS-CoV-2 M pro . Figure S2 : Fluorescence emission spectrum of SARS-CoV-2 M pro , recorded at an excitation of 292 nm.
Conflicts of Interest: The authors declare no conflicts of interest.
References
Abdelrahman, Li, Wang, Comparative Review of SARS-CoV-2, SARS-CoV, MERS-CoV, and Influenza A Respiratory Viruses, Front. Immunol, doi:10.3389/fimmu.2020.552909
Abian, Ortega-Alarcon, Jimenez-Alesanco, Ceballos-Laita, Vega et al., Structural stability of SARS-CoV-2 3CLpro and identification of quercetin as an inhibitor by experimental screening, Int. J. Biol. Macromol, doi:10.1016/j.ijbiomac.2020.07.235
Adegbola, Fadahunsi, Ogunjinmi, Adegbola, Ojeniyi et al., Potential inhibitory properties of structurally modified quercetin/isohamnetin glucosides against SARS-CoV-2 Mpro; molecular docking and dynamics simulation strategies, Inform. Med. Unlocked, doi:10.1016/j.imu.2023.101167
Alarabei, Aziz, Ab Razak, Abas, Bahari et al., Immunomodulating Phytochemicals: An Insight Into Their Potential Use in Cytokine Storm Situations, Adv. Pharm. Bull, doi:10.34172/apb.2024.001
Antonopoulou, Sapountzaki, Rova, Christakopoulos, Inhibition of the main protease of SARS-CoV-2 (M(pro)) by repurposing/designing drug-like substances and utilizing nature's toolbox of bioactive compounds, Comput. Struct. Biotechnol. J, doi:10.1016/j.csbj.2022.03.009
Baggieri, Gioacchini, Borgonovo, Catinella, Marchi et al., Antiviral, virucidal and antioxidant properties of Artemisia annua against SARS-CoV-2, Biomed. Pharmacother, doi:10.1016/j.biopha.2023.115682
Bano, Ahmedi, Manzoor, Dey, Advances in Antifungal Drug Development: Natural Products with Antifungal Potential
Behera, Singh, Subba, Mc, Sahu et al., Effectiveness of COVID-19 vaccine (Covaxin) against breakthrough SARS-CoV-2 infection in India, Hum. Vaccines Immunother, doi:10.1080/21645515.2022.2034456
Chen, Ding, Guan, Zhou, He et al., The Pharmacological Effects and Potential Applications of Limonene from Citrus Plants: A Review, Nat. Prod. Commun, doi:10.1177/1934578X241254229
Chhetri, Chettri, Rai, Mishra, Sinha et al., characterization and computational study on potential inhibitory action of novel azo imidazole derivatives against COVID-19 main protease (M(pro): 6LU7), J. Mol. Struct, doi:10.1016/j.molstruc.2020.129230
Courouble, Dey, Yadav, Timm, Harrison et al., Revealing the Structural Plasticity of SARS-CoV-2 nsp7 and nsp8 Using Structural Proteomics, J. Am. Soc. Mass Spectrom, doi:10.1021/jasms.1c00086
Das, Nath, Shahjahan; Dey, Plausible mechanism of drug resistance and side-effects of COVID-19 therapeutics: A bottleneck for its eradication, DARU J. Pharm. Sci, doi:10.1007/s40199-024-00524-z
Dey, Dey, SARS-CoV-2 pandemic, COVID-19 case fatality rates and deaths per million population in India, J. Bioinform. Comput. Syst. Biol
Dey, Saini, Dhembla, Bhatt, Rajesh et al., penciclovir, and anidulafungin exhibit potential in the treatment of COVID-19 via binding to nsp12 of SARS-CoV-2, J. Biomol. Struct. Dyn, doi:10.1080/07391102.2021.2000498
Elsebai, Albalawi, Essential Oils and COVID-19, Molecules, doi:10.3390/molecules27227893
Faddis, Du, Stewart, Hasan, Lewit et al., Molecular Modelling, Synthesis, and In-Vitro Assay to Identify Potential Antiviral Peptides Targeting the 3-Chymotrypsin-Like Protease of SARS-CoV-2, Int. J. Pept. Res. Ther, doi:10.1007/s10989-023-10563-w
Faksova, Walsh, Jiang, Griffin, Phillips et al., COVID-19 vaccines and adverse events of special interest: A multinational Global Vaccine Data Network (GVDN) cohort study of 99 million vaccinated individuals, Vaccine, doi:10.1016/j.vaccine.2024.01.100
Farasati Far, Bokov, Widjaja, Setia Budi, Kamal Abdelbasset et al., acyclovir and tetrahydrobiopterin may be promising to treat COVID-19 patients, through interaction with interleukin-12, J. Biomol. Struct. Dyn, doi:10.1080/07391102.2022.2064917
Forman, Ames, the Ames test, and the causes of cancer, BMJ, doi:10.1136/bmj.303.6800.428
Gao, Du, Qin, Wang, Du et al., Natural Small Molecule Drugs from Plants
Gao, Li, Zhang, Bai, Scopoletin: A review of its pharmacology, pharmacokinetics, and toxicity, Front. Pharmacol, doi:10.3389/fphar.2024.1268464
García-Montero, Fraile-Martínez, Bravo, Torres-Carranza, Sanchez-Trujillo et al., An Updated Review of SARS-CoV-2 Vaccines and the Importance of Effective Vaccination Programs in Pandemic Times, Vaccines, doi:10.3390/vaccines9050433
Gasmi, Mujawdiya, Lysiuk, Shanaida, Peana et al., Quercetin in the Prevention and Treatment of Coronavirus Infections: A Focus on SARS-CoV-2, Pharmaceuticals, doi:10.3390/ph15091049
Gehlen, The centenary of the Stern-Volmer equation of fluorescence quenching: From the single line plot to the SV quenching map, J. Photochem. Photobiol. C Photochem. Rev, doi:10.1016/j.jphotochemrev.2019.100338
Ihssen, Faccio, Yao, Sirec, Spitz, Fluorogenic in vitro activity assay for the main protease Mpro from SARS-CoV-2 and its adaptation to the identification of inhibitors, STAR Protoc, doi:10.1016/j.xpro.2021.100793
Ikanovic, Šeherčehajić, Saric Medic, Tomic, Hadžiselimović, In Silico Analysis of Scopoletin Interaction with Potential SARS-CoV-2 Target
Jangir, Dey, Kundu, Mehrotra, Assessment of amsacrine binding with DNA using UV-visible, circular dichroism and Raman spectroscopic techniques, J. Photochem. Photobiol. B Biol, doi:10.1016/j.jphotobiol.2012.05.005
Jin, Du, Xu, Deng, Liu et al., Structure of Mpro from SARS-CoV-2 and discovery of its inhibitors, Nature, doi:10.1038/s41586-020-2223-y
Kaul, Paul, Kumar, Büsselberg, Dwivedi et al., Promising Antiviral Activities of Natural Flavonoids against SARS-CoV-2 Targets: Systematic Review, Int. J. Mol. Sci, doi:10.3390/ijms222011069
Khan, Kang, Ali, Lai, Remdesivir Strongly Binds to RNA-Dependent RNA Polymerase, Membrane Protein, and Main Protease of SARS-CoV-2: Indication From Molecular Modeling and Simulations, Front. Pharmacol, doi:10.3389/fphar.2021.710778
Lemos, Florêncio, Pinto, Campos, Silva et al., Antifungal Activity of the Natural Coumarin Scopoletin Against Planktonic Cells and Biofilms From a Multidrug-Resistant Candida tropicalis Strain, Front. Microbiol, doi:10.3389/fmicb.2020.01525
Li, Liu, Li, Li, Li et al., Efficacy, immunogenicity and safety of COVID-19 vaccines in older adults: A systematic review and meta-analysis, Front. Immunol, doi:10.3389/fimmu.2022.965971
Loizzo, Saab, Tundis, Statti, Menichini et al., Phytochemical analysis and in vitro antiviral activities of the essential oils of seven Lebanon species, Chem. Biodivers, doi:10.1002/cbdv.200890045
Majchrzak, Madej, Łysek-Gładysi Ńska, Zarębska-Michaluk, Zegadło et al., The RdRp genotyping of SARS-CoV-2 isolated from patients with different clinical spectrum of COVID-19, BMC Infect. Dis, doi:10.1186/s12879-024-09146-x
Majerová, Konvalinka, Viral proteases as therapeutic targets, Mol. Asp. Med, doi:10.1016/j.mam.2022.101159
Mbaveng, Zhao, Kuete, 20-Harmful and Protective Effects of Phenolic Compounds from African Medicinal Plants
Mengist, Dilnessa, Jin, Structural Basis of Potential Inhibitors Targeting SARS-CoV-2 Main Protease, Front. Chem, doi:10.3389/fchem.2021.622898
Mohammad, Mathur, Hassan, InstaDock: A single-click graphical user interface for molecular docking-based virtual high-throughput screening, Brief. Bioinform, doi:10.1093/bib/bbaa279
Mátyus, Szöllősi, Jenei, Steady-state fluorescence quenching applications for studying protein structure and dynamics, J. Photochem. Photobiol. B Biol, doi:10.1016/j.jphotobiol.2005.12.017
Nanishi, Levy, Ozonoff, Waning effectiveness of SARS-CoV-2 mRNA vaccines in older adults: A rapid review, Hum. Vaccines Immunother, doi:10.1080/21645515.2022.2045857
Ng, Correia, Seagal, Degoey, Schrimpf et al., Antiviral Drug Discovery for the Treatment of COVID-19 Infections, Viruses, doi:10.3390/v14050961
Pang, Xu, Liu, Li, Chen, The research progress of SARS-CoV-2 main protease inhibitors from 2020 to 2022, Eur. J. Med. Chem, doi:10.1016/j.ejmech.2023.115491
Panikar, Shoba, Arun, Sahayarayan, Usha Raja Nanthini et al., Essential oils as an effective alternative for the treatment of COVID-19: Molecular interaction analysis of protease (Mpro) with pharmacokinetics and toxicological properties, J. Infect. Public Health, doi:10.1016/j.jiph.2020.12.037
Parmar, Thumar, Patel, Athar, Jha et al., Structural differences in 3C-like protease (Mpro) from SARS-CoV and SARS-CoV-2: Molecular insights revealed by Molecular Dynamics Simulations, Struct. Chem, doi:10.1007/s11224-022-02089-6
Pascetta, Investigating the Main Protease (MPro) of SARS-CoV-2 as a Potential Drug Target
Promdam, Panichayupakaranant, Gingerol: A narrative review of its beneficial effect on human health, Food Chem. Adv, doi:10.1016/j.focha.2022.100043
Riveiro, Kimpe, Moglioni, Vazquez, Monczor et al., Coumarins: Old Compounds with Novel Promising Therapeutic Perspectives, Curr. Med. Chem, doi:10.2174/092986710790936284
Rizwan, Kothidar, Meghwani, Sharma, Shobhawat et al., Comparative analysis of SARS-CoV-2 envelope viroporin mutations from COVID-19 deceased and surviving patients revealed implications on its ion-channel activities and correlation with patient mortality, J. Biomol. Struct. Dyn, doi:10.1080/07391102.2021.1944319
Rut, Groborz, Zhang, Sun, Zmudzinski et al., SARS-CoV-2 Mpro inhibitors and activity-based probes for patient-sample imaging, Nat. Chem. Biol, doi:10.1038/s41589-020-00689-z
Rut, Groborz, Zhang, Sun, Zmudzinski et al., Substrate specificity profiling of SARS-CoV-2 Mpro protease provides basis for anti-COVID-19 drug design, BioRxiv, doi:10.1101/2020.03.07.981928
Salehi, Upadhyay, Erdogan Orhan, Kumar Jugran, Jayaweera et al., Therapeutic Potential of αand β-Pinene: A Miracle Gift of Nature, Biomolecules, doi:10.3390/biom9110738
Shamsi, Al Shahwan, Ahamad, Hassan, Ahmad et al., Spectroscopic, calorimetric and molecular docking insight into the interaction of Alzheimer's drug donepezil with human transferrin: Implications of Alzheimer's drug, J. Biomol. Struct. Dyn, doi:10.1080/07391102.2019.1595728
Singh, Singhal, Pandey, Amit; Mallik, Dubey, Phytochemical Investigation on Tinospora cordifolia and Alstonia scholaris, Res. J. Pharm. Technol, doi:10.52711/0974-360X.2024.00421
Surendran, Qassadi, Surendran, Lilley, Heinrich, Myrcene-What Are the Potential Health Benefits of This Flavouring and Aroma Agent?, Front. Nutr, doi:10.3389/fnut.2021.699666
Tao, Zhang, Du, Liao, Cai et al., Allosteric inhibition of SARS-CoV-2 3CL protease by colloidal bismuth subcitrate, Chem. Sci, doi:10.1039/D1SC03526F
Teli, Balar, Patel, Sharma, Chavda et al., Molnupiravir: A Versatile Prodrug against SARS-CoV-2 Variants, Metabolites, doi:10.3390/metabo13020309
Teo, Review of COVID-19 Vaccines and Their Evidence in Older Adults, Ann. Geriatr. Med. Res, doi:10.4235/agmr.21.0011
Torres Neto, Monteiro, Fernández-Romero, Teleshova, Sailer et al., Essential oils block cellular entry of SARS-CoV-2 delta variant, Sci. Rep, doi:10.1038/s41598-022-25342-8
Waseem, Anwar, Khan, Shamsi, Hassan et al., MAP/Microtubule Affinity Regulating Kinase 4 Inhibitory Potential of Irisin: A New Therapeutic Strategy to Combat Cancer and Alzheimer's Disease, Int. J. Mol. Sci, doi:10.3390/ijms222010986
Waseem, Shamsi, Khan, Hassan, Kazim et al., Unraveling the Binding Mechanism of Alzheimer's Drugs with Irisin: Spectroscopic, Calorimetric, and Computational Approaches, Int. J. Mol. Sci, doi:10.3390/ijms23115965
Wilkinson, Joffrin, Lebarbenchon, Mavingui, Partial RdRp sequences offer a robust method for Coronavirus subgenus classification, bioRxiv, doi:10.1101/2020.03.02.974311
Xu, Zhong, Yang, Fu, Shi et al., Quercetin possesses a fluorescence quenching effect but is a weak inhibitor against SARS-CoV-2 main protease, Proc. Natl. Acad. Sci, doi:10.1073/pnas.2309870120
Yadav, Courouble, Dey, Harrison, Timm et al., Biochemical and structural insights into SARS-CoV-2 polyprotein processing by Mpro, Sci. Adv, doi:10.1126/sciadv.add2191
Yadav, Mahalvar, Pradhan, Yadav, Kumar Sahu et al., Exploring the potential of phytochemicals and nanomaterial: A boon to antimicrobial treatment, Med. Drug Discov, doi:10.1016/j.medidd.2023.100151
Yammine, Gao, Kwan, Tryptophan Fluorescence Quenching Assays for Measuring Protein-ligand Binding Affinities: Principles and a Practical Guide, Bio-Protoc, doi:10.21769/BioProtoc.3253
Yan, Zhang, Liu, Wang, Chen, Reframing quercetin as a promiscuous inhibitor against SARS-CoV-2 main protease, Proc. Natl. Acad. Sci, doi:10.1073/pnas.2309289120
Yang, Rao, Structural biology of SARS-CoV-2 and implications for therapeutic development, Nat. Rev. Microbiol, doi:10.1038/s41579-021-00630-8
Yuan, Wang, Wang, Chen, Ke et al., Network pharmacology and molecular docking reveal the mechanism of Scopoletin against non-small cell lung cancer, Life Sci, doi:10.1016/j.lfs.2021.119105
Zagórska, Czopek, Fryc, Jo Ńczyk, Inhibitors of SARS-CoV-2 Main Protease (Mpro) as Anti-Coronavirus Agents, Biomolecules, doi:10.3390/biom14070797
Zhang, Lin, Sun, Curth, Drosten et al., Crystal structure of SARS-CoV-2 main protease provides a basis for design of improved α-ketoamide inhibitors, Science, doi:10.1126/science.abb3405
Zhang, Xiang, Huo, Zhou, Jiang et al., Molecular mechanism of interaction between SARS-CoV-2 and host cells and interventional therapy, Signal Transduct. Target. Ther, doi:10.1038/s41392-021-00653-w
DOI record:
{
"DOI": "10.3390/v17030402",
"ISSN": [
"1999-4915"
],
"URL": "http://dx.doi.org/10.3390/v17030402",
"abstract": "<jats:p>The main protease (Mpro or 3CLpro or nsp5) of SARS-CoV-2 is crucial to the life cycle and pathogenesis of SARS-CoV-2, making it an attractive drug target to develop antivirals. This study employed the virtual screening of a few phytochemicals, and the resultant best compound, Scopoletin, was further investigated by a FRET-based enzymatic assay, revealing an experimental IC50 of 15.75 µM. The impact of Scopoletin on Mpro was further investigated by biophysical and MD simulation studies. Fluorescence spectroscopy identified a strong binding constant of 3.17 × 104 M⁻1 for Scopoletin binding to Mpro, as demonstrated by its effective fluorescence quenching of Mpro. Additionally, CD spectroscopy showed a significant reduction in the helical content of Mpro upon interaction with Scopoletin. The findings of thermodynamic measurements using isothermal titration calorimetry (ITC) supported the spectroscopic data, indicating a tight binding of Scopoletin to Mpro with a KA of 2.36 × 103 M−1. Similarly, interaction studies have also revealed that Scopoletin forms hydrogen bonds with the amino acids nearest to the active site, and this has been further supported by molecular dynamics simulation studies. These findings indicate that Scopoletin may be developed as a potential antiviral treatment for SARS-CoV-2 by targeting Mpro.</jats:p>",
"alternative-id": [
"v17030402"
],
"author": [
{
"ORCID": "https://orcid.org/0000-0001-6990-9528",
"affiliation": [
{
"name": "Laboratory for Proteins and Structural Biology, Dr. B.R. Ambedkar Center for Biomedical Research, University of Delhi, Delhi 110007, India"
}
],
"authenticated-orcid": false,
"family": "Bano",
"given": "Sarika",
"sequence": "first"
},
{
"ORCID": "https://orcid.org/0000-0001-9472-1922",
"affiliation": [
{
"name": "Laboratory for Proteins and Structural Biology, Dr. B.R. Ambedkar Center for Biomedical Research, University of Delhi, Delhi 110007, India"
}
],
"authenticated-orcid": false,
"family": "Singh",
"given": "Jyotishna",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0002-5387-3863",
"affiliation": [
{
"name": "Centre for Interdisciplinary Research in Basic Sciences, Jamia Millia Islamia, Jamia Nagar, New Delhi 110025, India"
}
],
"authenticated-orcid": false,
"family": "Zehra",
"given": "Zainy",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0009-0004-0255-7117",
"affiliation": [
{
"name": "Centre for Interdisciplinary Research in Basic Sciences, Jamia Millia Islamia, Jamia Nagar, New Delhi 110025, India"
}
],
"authenticated-orcid": false,
"family": "Sulaimani",
"given": "Md Nayab",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0002-0399-4835",
"affiliation": [
{
"name": "Centre for Interdisciplinary Research in Basic Sciences, Jamia Millia Islamia, Jamia Nagar, New Delhi 110025, India"
}
],
"authenticated-orcid": false,
"family": "Mohammad",
"given": "Taj",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0009-0005-3911-6240",
"affiliation": [
{
"name": "Laboratory for Proteins, Dr. B.R. Ambedkar Center for Biomedical Research, University of Delhi, Delhi 110007, India"
}
],
"authenticated-orcid": false,
"family": "Yumlembam",
"given": "Seemasundari",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0002-3663-4940",
"affiliation": [
{
"name": "Centre for Interdisciplinary Research in Basic Sciences, Jamia Millia Islamia, Jamia Nagar, New Delhi 110025, India"
}
],
"authenticated-orcid": false,
"family": "Hassan",
"given": "Md Imtaiyaz",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0001-9060-7970",
"affiliation": [
{
"name": "Centre for Interdisciplinary Research in Basic Sciences, Jamia Millia Islamia, Jamia Nagar, New Delhi 110025, India"
}
],
"authenticated-orcid": false,
"family": "Islam",
"given": "Asimul",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0001-7062-9574",
"affiliation": [
{
"name": "Laboratory for Proteins and Structural Biology, Dr. B.R. Ambedkar Center for Biomedical Research, University of Delhi, Delhi 110007, India"
}
],
"authenticated-orcid": false,
"family": "Dey",
"given": "Sanjay Kumar",
"sequence": "additional"
}
],
"container-title": "Viruses",
"container-title-short": "Viruses",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2025,
3,
12
]
],
"date-time": "2025-03-12T11:31:42Z",
"timestamp": 1741779102000
},
"deposited": {
"date-parts": [
[
2025,
3,
12
]
],
"date-time": "2025-03-12T11:46:16Z",
"timestamp": 1741779976000
},
"funder": [
{
"name": "Dr. B.R. Ambedkar Center for Biomedical Research, University of Delhi"
},
{
"award": [
"IoE/2021/12/FRP"
],
"name": "The Institution of Eminence"
}
],
"indexed": {
"date-parts": [
[
2025,
3,
13
]
],
"date-time": "2025-03-13T04:16:41Z",
"timestamp": 1741839401050,
"version": "3.38.0"
},
"is-referenced-by-count": 0,
"issue": "3",
"issued": {
"date-parts": [
[
2025,
3,
12
]
]
},
"journal-issue": {
"issue": "3",
"published-online": {
"date-parts": [
[
2025,
3
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2025,
3,
12
]
],
"date-time": "2025-03-12T00:00:00Z",
"timestamp": 1741737600000
}
}
],
"link": [
{
"URL": "https://www.mdpi.com/1999-4915/17/3/402/pdf",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "1968",
"original-title": [],
"page": "402",
"prefix": "10.3390",
"published": {
"date-parts": [
[
2025,
3,
12
]
]
},
"published-online": {
"date-parts": [
[
2025,
3,
12
]
]
},
"publisher": "MDPI AG",
"reference": [
{
"DOI": "10.1016/j.ejmech.2023.115491",
"article-title": "The research progress of SARS-CoV-2 main protease inhibitors from 2020 to 2022",
"author": "Pang",
"doi-asserted-by": "crossref",
"first-page": "115491",
"journal-title": "Eur. J. Med. Chem.",
"key": "ref_1",
"volume": "257",
"year": "2023"
},
{
"DOI": "10.3389/fimmu.2020.552909",
"doi-asserted-by": "crossref",
"key": "ref_2",
"unstructured": "Abdelrahman, Z., Li, M., and Wang, X. (2020). Comparative Review of SARS-CoV-2, SARS-CoV, MERS-CoV, and Influenza A Respiratory Viruses. Front. Immunol., 11."
},
{
"key": "ref_3",
"unstructured": "Dey, J.K., and Dey, S.K. (2020). SARS-CoV-2 pandemic, COVID-19 case fatality rates and deaths per million population in India. J. Bioinform. Comput. Syst. Biol., 2."
},
{
"DOI": "10.1038/s41579-021-00630-8",
"article-title": "Structural biology of SARS-CoV-2 and implications for therapeutic development",
"author": "Yang",
"doi-asserted-by": "crossref",
"first-page": "685",
"journal-title": "Nat. Rev. Microbiol.",
"key": "ref_4",
"volume": "19",
"year": "2021"
},
{
"DOI": "10.1039/D1SC03526F",
"article-title": "Allosteric inhibition of SARS-CoV-2 3CL protease by colloidal bismuth subcitrate",
"author": "Tao",
"doi-asserted-by": "crossref",
"first-page": "14098",
"journal-title": "Chem. Sci.",
"key": "ref_5",
"volume": "12",
"year": "2021"
},
{
"DOI": "10.1126/sciadv.add2191",
"article-title": "Biochemical and structural insights into SARS-CoV-2 polyprotein processing by Mpro",
"author": "Yadav",
"doi-asserted-by": "crossref",
"first-page": "eadd2191",
"journal-title": "Sci. Adv.",
"key": "ref_6",
"volume": "8",
"year": "2022"
},
{
"DOI": "10.1021/jasms.1c00086",
"article-title": "Revealing the Structural Plasticity of SARS-CoV-2 nsp7 and nsp8 Using Structural Proteomics",
"author": "Courouble",
"doi-asserted-by": "crossref",
"first-page": "1618",
"journal-title": "J. Am. Soc. Mass Spectrom.",
"key": "ref_7",
"volume": "32",
"year": "2021"
},
{
"DOI": "10.1016/j.molstruc.2020.129230",
"article-title": "Synthesis, characterization and computational study on potential inhibitory action of novel azo imidazole derivatives against COVID-19 main protease (M(pro): 6LU7)",
"author": "Chhetri",
"doi-asserted-by": "crossref",
"first-page": "129230",
"journal-title": "J. Mol. Struct.",
"key": "ref_8",
"volume": "1225",
"year": "2021"
},
{
"DOI": "10.1038/s41392-021-00653-w",
"article-title": "Molecular mechanism of interaction between SARS-CoV-2 and host cells and interventional therapy",
"author": "Zhang",
"doi-asserted-by": "crossref",
"first-page": "233",
"journal-title": "Signal Transduct. Target. Ther.",
"key": "ref_9",
"volume": "6",
"year": "2021"
},
{
"DOI": "10.1007/s10989-023-10563-w",
"article-title": "Molecular Modelling, Synthesis, and In-Vitro Assay to Identify Potential Antiviral Peptides Targeting the 3-Chymotrypsin-Like Protease of SARS-CoV-2",
"author": "Faddis",
"doi-asserted-by": "crossref",
"first-page": "89",
"journal-title": "Int. J. Pept. Res. Ther.",
"key": "ref_10",
"volume": "29",
"year": "2023"
},
{
"DOI": "10.1038/s41586-020-2223-y",
"article-title": "Structure of Mpro from SARS-CoV-2 and discovery of its inhibitors",
"author": "Jin",
"doi-asserted-by": "crossref",
"first-page": "289",
"journal-title": "Nature",
"key": "ref_11",
"volume": "582",
"year": "2020"
},
{
"DOI": "10.3389/fchem.2021.622898",
"doi-asserted-by": "crossref",
"key": "ref_12",
"unstructured": "Mengist, H.M., Dilnessa, T., and Jin, T. (2021). Structural Basis of Potential Inhibitors Targeting SARS-CoV-2 Main Protease. Front. Chem., 9."
},
{
"DOI": "10.3390/vaccines9050433",
"doi-asserted-by": "crossref",
"key": "ref_13",
"unstructured": "García-Montero, C., Fraile-Martínez, O., Bravo, C., Torres-Carranza, D., Sanchez-Trujillo, L., Gómez-Lahoz, A.M., Guijarro, L.G., García-Honduvilla, N., Asúnsolo, A., and Bujan, J. (2021). An Updated Review of SARS-CoV-2 Vaccines and the Importance of Effective Vaccination Programs in Pandemic Times. Vaccines, 9."
},
{
"DOI": "10.1016/j.vaccine.2024.01.100",
"article-title": "COVID-19 vaccines and adverse events of special interest: A multinational Global Vaccine Data Network (GVDN) cohort study of 99 million vaccinated individuals",
"author": "Faksova",
"doi-asserted-by": "crossref",
"first-page": "2200",
"journal-title": "Vaccine",
"key": "ref_14",
"volume": "42",
"year": "2024"
},
{
"DOI": "10.1080/21645515.2022.2034456",
"article-title": "Effectiveness of COVID-19 vaccine (Covaxin) against breakthrough SARS-CoV-2 infection in India",
"author": "Behera",
"doi-asserted-by": "crossref",
"first-page": "2034456",
"journal-title": "Hum. Vaccines Immunother.",
"key": "ref_15",
"volume": "18",
"year": "2022"
},
{
"DOI": "10.1007/s40199-024-00524-z",
"article-title": "Plausible mechanism of drug resistance and side-effects of COVID-19 therapeutics: A bottleneck for its eradication",
"author": "Das",
"doi-asserted-by": "crossref",
"first-page": "801",
"journal-title": "DARU J. Pharm. Sci.",
"key": "ref_16",
"volume": "32",
"year": "2024"
},
{
"DOI": "10.3389/fimmu.2022.965971",
"article-title": "Efficacy, immunogenicity and safety of COVID-19 vaccines in older adults: A systematic review and meta-analysis",
"author": "Li",
"doi-asserted-by": "crossref",
"first-page": "965971",
"journal-title": "Front. Immunol.",
"key": "ref_17",
"volume": "13",
"year": "2022"
},
{
"DOI": "10.4235/agmr.21.0011",
"article-title": "Review of COVID-19 Vaccines and Their Evidence in Older Adults",
"author": "Teo",
"doi-asserted-by": "crossref",
"first-page": "4",
"journal-title": "Ann. Geriatr. Med. Res.",
"key": "ref_18",
"volume": "25",
"year": "2021"
},
{
"DOI": "10.1080/21645515.2022.2045857",
"article-title": "Waning effectiveness of SARS-CoV-2 mRNA vaccines in older adults: A rapid review",
"author": "Nanishi",
"doi-asserted-by": "crossref",
"first-page": "2045857",
"journal-title": "Hum. Vaccines Immunother.",
"key": "ref_19",
"volume": "18",
"year": "2022"
},
{
"DOI": "10.3390/v14050961",
"doi-asserted-by": "crossref",
"key": "ref_20",
"unstructured": "Ng, T.I., Correia, I., Seagal, J., DeGoey, D.A., Schrimpf, M.R., Hardee, D.J., Noey, E.L., and Kati, W.M. (2022). Antiviral Drug Discovery for the Treatment of COVID-19 Infections. Viruses, 14."
},
{
"DOI": "10.1016/j.mam.2022.101159",
"article-title": "Viral proteases as therapeutic targets",
"author": "Konvalinka",
"doi-asserted-by": "crossref",
"first-page": "101159",
"journal-title": "Mol. Asp. Med.",
"key": "ref_21",
"volume": "88",
"year": "2022"
},
{
"DOI": "10.3390/metabo13020309",
"doi-asserted-by": "crossref",
"key": "ref_22",
"unstructured": "Teli, D., Balar, P., Patel, K., Sharma, A., Chavda, V., and Vora, L. (2023). Molnupiravir: A Versatile Prodrug against SARS-CoV-2 Variants. Metabolites, 13."
},
{
"DOI": "10.3389/fphar.2021.710778",
"doi-asserted-by": "crossref",
"key": "ref_23",
"unstructured": "Khan, F.I., Kang, T., Ali, H., and Lai, D. (2021). Remdesivir Strongly Binds to RNA-Dependent RNA Polymerase, Membrane Protein, and Main Protease of SARS-CoV-2: Indication From Molecular Modeling and Simulations. Front. Pharmacol., 12."
},
{
"DOI": "10.1126/science.abb3405",
"article-title": "Crystal structure of SARS-CoV-2 main protease provides a basis for design of improved α-ketoamide inhibitors",
"author": "Zhang",
"doi-asserted-by": "crossref",
"first-page": "409",
"journal-title": "Science",
"key": "ref_24",
"volume": "368",
"year": "2020"
},
{
"DOI": "10.1101/2020.03.02.974311",
"doi-asserted-by": "crossref",
"key": "ref_25",
"unstructured": "Wilkinson, D.A., Joffrin, L., Lebarbenchon, C., and Mavingui, P. (2020). Partial RdRp sequences offer a robust method for Coronavirus subgenus classification. bioRxiv."
},
{
"DOI": "10.1186/s12879-024-09146-x",
"doi-asserted-by": "crossref",
"key": "ref_26",
"unstructured": "Majchrzak, M., Madej, Ł., Łysek-Gładysińska, M., Zarębska-Michaluk, D., Zegadło, K., Dziuba, A., Nogal-Nowak, K., Kondziołka, W., Sufin, I., and Myszona-Tarnowska, M. (2024). The RdRp genotyping of SARS-CoV-2 isolated from patients with different clinical spectrum of COVID-19. BMC Infect. Dis., 24."
},
{
"DOI": "10.1007/978-981-97-5165-5",
"doi-asserted-by": "crossref",
"key": "ref_27",
"unstructured": "Manzoor, N. (2024). Toxicology of Antifungal and Antiviral Drugs. Advances in Antifungal Drug Development: Natural Products with Antifungal Potential, Springer Nature Singapore."
},
{
"DOI": "10.3390/biom14070797",
"doi-asserted-by": "crossref",
"key": "ref_28",
"unstructured": "Zagórska, A., Czopek, A., Fryc, M., and Jończyk, J. (2024). Inhibitors of SARS-CoV-2 Main Protease (Mpro) as Anti-Coronavirus Agents. Biomolecules, 14."
},
{
"DOI": "10.52711/0974-360X.2024.00421",
"article-title": "Phytochemical Investigation on Tinospora cordifolia and Alstonia scholaris",
"author": "Singh",
"doi-asserted-by": "crossref",
"first-page": "2689",
"journal-title": "Res. J. Pharm. Technol.",
"key": "ref_29",
"volume": "17",
"year": "2024"
},
{
"DOI": "10.1007/978-981-10-8022-7_55",
"doi-asserted-by": "crossref",
"key": "ref_30",
"unstructured": "Gao, L., Du, L.D., Qin, X.M., Wang, J.H., and Du, G.H. (2018). Strychnine. Natural Small Molecule Drugs from Plants, Springer."
},
{
"DOI": "10.1016/j.focha.2022.100043",
"article-title": "[6]-Gingerol: A narrative review of its beneficial effect on human health",
"author": "Promdam",
"doi-asserted-by": "crossref",
"first-page": "100043",
"journal-title": "Food Chem. Adv.",
"key": "ref_31",
"volume": "1",
"year": "2022"
},
{
"DOI": "10.3390/biom9110738",
"doi-asserted-by": "crossref",
"key": "ref_32",
"unstructured": "Salehi, B., Upadhyay, S., Erdogan Orhan, I., Kumar Jugran, A., Jayaweera, S.L.D., Dias, D.A., Sharopov, F., Taheri, Y., Martins, N., and Baghalpour, N. (2019). Therapeutic Potential of α- and β-Pinene: A Miracle Gift of Nature. Biomolecules, 9."
},
{
"article-title": "The Pharmacological Effects and Potential Applications of Limonene from Citrus Plants: A Review",
"author": "Chen",
"first-page": "1",
"journal-title": "Nat. Prod. Commun.",
"key": "ref_33",
"volume": "19",
"year": "2024"
},
{
"DOI": "10.1038/s41598-022-25342-8",
"doi-asserted-by": "crossref",
"key": "ref_34",
"unstructured": "Torres Neto, L., Monteiro, M.L.G., Fernández-Romero, J., Teleshova, N., Sailer, J., and Conte Junior, C.A. (2022). Essential oils block cellular entry of SARS-CoV-2 delta variant. Sci. Rep., 12."
},
{
"DOI": "10.1002/cbdv.200890045",
"article-title": "Phytochemical analysis and in vitro antiviral activities of the essential oils of seven Lebanon species",
"author": "Loizzo",
"doi-asserted-by": "crossref",
"first-page": "461",
"journal-title": "Chem. Biodivers.",
"key": "ref_35",
"volume": "5",
"year": "2008"
},
{
"DOI": "10.3389/fnut.2021.699666",
"doi-asserted-by": "crossref",
"key": "ref_36",
"unstructured": "Surendran, S., Qassadi, F., Surendran, G., Lilley, D., and Heinrich, M. (2021). Myrcene-What Are the Potential Health Benefits of This Flavouring and Aroma Agent?. Front. Nutr., 8."
},
{
"DOI": "10.1007/978-3-030-75275-0_99",
"doi-asserted-by": "crossref",
"key": "ref_37",
"unstructured": "Ikanovic, T., Šeherčehajić, E., Saric Medic, B., Tomic, N., and Hadžiselimović, R. (2021). In Silico Analysis of Scopoletin Interaction with Potential SARS-CoV-2 Target. International Conference “New Technologies, Development and Applications”, Springer International Publishing."
},
{
"DOI": "10.1016/j.biopha.2023.115682",
"doi-asserted-by": "crossref",
"key": "ref_38",
"unstructured": "Baggieri, M., Gioacchini, S., Borgonovo, G., Catinella, G., Marchi, A., Picone, P., Vasto, S., Fioravanti, R., Bucci, P., and Kojouri, M. (2023). Antiviral, virucidal and antioxidant properties of Artemisia annua against SARS-CoV-2. Biomed. Pharmacother., 168."
},
{
"article-title": "Immunomodulating Phytochemicals: An Insight Into Their Potential Use in Cytokine Storm Situations",
"author": "Alarabei",
"first-page": "105",
"journal-title": "Adv. Pharm. Bull.",
"key": "ref_39",
"volume": "14",
"year": "2024"
},
{
"DOI": "10.1016/j.medidd.2023.100151",
"article-title": "Exploring the potential of phytochemicals and nanomaterial: A boon to antimicrobial treatment",
"author": "Yadav",
"doi-asserted-by": "crossref",
"first-page": "100151",
"journal-title": "Med. Drug Discov.",
"key": "ref_40",
"volume": "17",
"year": "2023"
},
{
"DOI": "10.3389/fphar.2024.1268464",
"doi-asserted-by": "crossref",
"key": "ref_41",
"unstructured": "Gao, X.Y., Li, X.Y., Zhang, C.Y., and Bai, C.Y. (2024). Scopoletin: A review of its pharmacology, pharmacokinetics, and toxicity. Front. Pharmacol., 15."
},
{
"DOI": "10.1016/j.jiph.2020.12.037",
"article-title": "Essential oils as an effective alternative for the treatment of COVID-19: Molecular interaction analysis of protease (Mpro) with pharmacokinetics and toxicological properties",
"author": "Panikar",
"doi-asserted-by": "crossref",
"first-page": "601",
"journal-title": "J. Infect. Public Health",
"key": "ref_42",
"volume": "14",
"year": "2021"
},
{
"DOI": "10.3390/molecules27227893",
"doi-asserted-by": "crossref",
"key": "ref_43",
"unstructured": "Elsebai, M.F., and Albalawi, M.A. (2022). Essential Oils and COVID-19. Molecules, 27."
},
{
"DOI": "10.1016/j.imu.2023.101167",
"article-title": "Potential inhibitory properties of structurally modified quercetin/isohamnetin glucosides against SARS-CoV-2 Mpro; molecular docking and dynamics simulation strategies",
"author": "Adegbola",
"doi-asserted-by": "crossref",
"first-page": "101167",
"journal-title": "Inform. Med. Unlocked",
"key": "ref_44",
"volume": "37",
"year": "2023"
},
{
"DOI": "10.3390/ph15091049",
"doi-asserted-by": "crossref",
"key": "ref_45",
"unstructured": "Gasmi, A., Mujawdiya, P.K., Lysiuk, R., Shanaida, M., Peana, M., Gasmi Benahmed, A., Beley, N., Kovalska, N., and Bjørklund, G. (2022). Quercetin in the Prevention and Treatment of Coronavirus Infections: A Focus on SARS-CoV-2. Pharmaceuticals, 15."
},
{
"DOI": "10.1016/j.ijbiomac.2020.07.235",
"article-title": "Structural stability of SARS-CoV-2 3CLpro and identification of quercetin as an inhibitor by experimental screening",
"author": "Abian",
"doi-asserted-by": "crossref",
"first-page": "1693",
"journal-title": "Int. J. Biol. Macromol.",
"key": "ref_46",
"volume": "164",
"year": "2020"
},
{
"DOI": "10.1093/bib/bbaa279",
"doi-asserted-by": "crossref",
"key": "ref_47",
"unstructured": "Mohammad, T., Mathur, Y., and Hassan, M.I. (2021). InstaDock: A single-click graphical user interface for molecular docking-based virtual high-throughput screening. Brief. Bioinform., 22."
},
{
"key": "ref_48",
"unstructured": "Rut, W., Groborz, K., Zhang, L., Sun, X., Zmudzinski, M., Hilgenfeld, R., and Drag, M. (2020). Substrate specificity profiling of SARS-CoV-2 Mpro protease provides basis for anti-COVID-19 drug design. BioRxiv."
},
{
"DOI": "10.1038/s41589-020-00689-z",
"article-title": "SARS-CoV-2 Mpro inhibitors and activity-based probes for patient-sample imaging",
"author": "Rut",
"doi-asserted-by": "crossref",
"first-page": "222",
"journal-title": "Nat. Chem. Biol.",
"key": "ref_49",
"volume": "17",
"year": "2021"
},
{
"DOI": "10.1016/j.xpro.2021.100793",
"article-title": "Fluorogenic in vitro activity assay for the main protease Mpro from SARS-CoV-2 and its adaptation to the identification of inhibitors",
"author": "Ihssen",
"doi-asserted-by": "crossref",
"first-page": "100793",
"journal-title": "STAR Protoc.",
"key": "ref_50",
"volume": "2",
"year": "2021"
},
{
"DOI": "10.1073/pnas.2309870120",
"article-title": "Reply to Yan et al.: Quercetin possesses a fluorescence quenching effect but is a weak inhibitor against SARS-CoV-2 main protease",
"author": "Xu",
"doi-asserted-by": "crossref",
"first-page": "e2309870120",
"journal-title": "Proc. Natl. Acad. Sci. USA",
"key": "ref_51",
"volume": "120",
"year": "2023"
},
{
"DOI": "10.1073/pnas.2309289120",
"article-title": "Reframing quercetin as a promiscuous inhibitor against SARS-CoV-2 main protease",
"author": "Yan",
"doi-asserted-by": "crossref",
"first-page": "e2309289120",
"journal-title": "Proc. Natl. Acad. Sci. USA",
"key": "ref_52",
"volume": "120",
"year": "2023"
},
{
"DOI": "10.3390/ijms222011069",
"doi-asserted-by": "crossref",
"key": "ref_53",
"unstructured": "Kaul, R., Paul, P., Kumar, S., Büsselberg, D., Dwivedi, V.D., and Chaari, A. (2021). Promising Antiviral Activities of Natural Flavonoids against SARS-CoV-2 Targets: Systematic Review. Int. J. Mol. Sci., 22."
},
{
"DOI": "10.1080/07391102.2021.1944319",
"article-title": "Comparative analysis of SARS-CoV-2 envelope viroporin mutations from COVID-19 deceased and surviving patients revealed implications on its ion-channel activities and correlation with patient mortality",
"author": "Rizwan",
"doi-asserted-by": "crossref",
"first-page": "10454",
"journal-title": "J. Biomol. Struct. Dyn.",
"key": "ref_54",
"volume": "40",
"year": "2022"
},
{
"DOI": "10.1080/07391102.2022.2064917",
"article-title": "Metronidazole, acyclovir and tetrahydrobiopterin may be promising to treat COVID-19 patients, through interaction with interleukin-12",
"author": "Bokov",
"doi-asserted-by": "crossref",
"first-page": "4253",
"journal-title": "J. Biomol. Struct. Dyn.",
"key": "ref_55",
"volume": "41",
"year": "2023"
},
{
"DOI": "10.1080/07391102.2021.2000498",
"article-title": "Suramin, penciclovir, and anidulafungin exhibit potential in the treatment of COVID-19 via binding to nsp12 of SARS-CoV-2",
"author": "Dey",
"doi-asserted-by": "crossref",
"first-page": "14067",
"journal-title": "J. Biomol. Struct. Dyn.",
"key": "ref_56",
"volume": "40",
"year": "2022"
},
{
"DOI": "10.3390/ijms23115965",
"doi-asserted-by": "crossref",
"key": "ref_57",
"unstructured": "Waseem, R., Shamsi, A., Khan, T., Hassan, M.I., Kazim, S.N., Shahid, M., and Islam, A. (2022). Unraveling the Binding Mechanism of Alzheimer’s Drugs with Irisin: Spectroscopic, Calorimetric, and Computational Approaches. Int. J. Mol. Sci., 23."
},
{
"DOI": "10.3390/ijms222010986",
"doi-asserted-by": "crossref",
"key": "ref_58",
"unstructured": "Waseem, R., Anwar, S., Khan, S., Shamsi, A., Hassan, M.I., Anjum, F., Shafie, A., Islam, A., and Yadav, D.K. (2021). MAP/Microtubule Affinity Regulating Kinase 4 Inhibitory Potential of Irisin: A New Therapeutic Strategy to Combat Cancer and Alzheimer’s Disease. Int. J. Mol. Sci., 22."
},
{
"DOI": "10.1016/j.jphotochemrev.2019.100338",
"doi-asserted-by": "crossref",
"key": "ref_59",
"unstructured": "Gehlen, M.H. (2020). The centenary of the Stern-Volmer equation of fluorescence quenching: From the single line plot to the SV quenching map. J. Photochem. Photobiol. C Photochem. Rev., 42."
},
{
"DOI": "10.1080/07391102.2019.1595728",
"article-title": "Spectroscopic, calorimetric and molecular docking insight into the interaction of Alzheimer’s drug donepezil with human transferrin: Implications of Alzheimer’s drug",
"author": "Shamsi",
"doi-asserted-by": "crossref",
"first-page": "1094",
"journal-title": "J. Biomol. Struct. Dyn.",
"key": "ref_60",
"volume": "38",
"year": "2020"
},
{
"key": "ref_61",
"unstructured": "Pascetta, V.G. (2022). Investigating the Main Protease (MPro) of SARS-CoV-2 as a Potential Drug Target. [Bachelor’s Thesis, University of New Hampshire]."
},
{
"DOI": "10.21769/BioProtoc.3253",
"doi-asserted-by": "crossref",
"key": "ref_62",
"unstructured": "Yammine, A., Gao, J., and Kwan, A.H. (2019). Tryptophan Fluorescence Quenching Assays for Measuring Protein-ligand Binding Affinities: Principles and a Practical Guide. Bio-Protoc., 9."
},
{
"DOI": "10.1016/j.jphotobiol.2005.12.017",
"article-title": "Steady-state fluorescence quenching applications for studying protein structure and dynamics",
"author": "Jenei",
"doi-asserted-by": "crossref",
"first-page": "223",
"journal-title": "J. Photochem. Photobiol. B Biol.",
"key": "ref_63",
"volume": "83",
"year": "2006"
},
{
"DOI": "10.1136/bmj.303.6800.428",
"article-title": "Ames, the Ames test, and the causes of cancer",
"author": "Forman",
"doi-asserted-by": "crossref",
"first-page": "428",
"journal-title": "BMJ",
"key": "ref_64",
"volume": "303",
"year": "1991"
},
{
"DOI": "10.1016/j.jphotobiol.2012.05.005",
"article-title": "Assessment of amsacrine binding with DNA using UV–visible, circular dichroism and Raman spectroscopic techniques",
"author": "Jangir",
"doi-asserted-by": "crossref",
"first-page": "38",
"journal-title": "J. Photochem. Photobiol. B Biol.",
"key": "ref_65",
"volume": "114",
"year": "2012"
},
{
"DOI": "10.1007/s11224-022-02089-6",
"article-title": "Structural differences in 3C-like protease (Mpro) from SARS-CoV and SARS-CoV-2: Molecular insights revealed by Molecular Dynamics Simulations",
"author": "Parmar",
"doi-asserted-by": "crossref",
"first-page": "1309",
"journal-title": "Struct. Chem.",
"key": "ref_66",
"volume": "34",
"year": "2023"
},
{
"DOI": "10.1016/j.csbj.2022.03.009",
"article-title": "Inhibition of the main protease of SARS-CoV-2 (M(pro)) by repurposing/designing drug-like substances and utilizing nature’s toolbox of bioactive compounds",
"author": "Antonopoulou",
"doi-asserted-by": "crossref",
"first-page": "1306",
"journal-title": "Comput. Struct. Biotechnol. J.",
"key": "ref_67",
"volume": "20",
"year": "2022"
},
{
"DOI": "10.2174/092986710790936284",
"article-title": "Coumarins: Old Compounds with Novel Promising Therapeutic Perspectives",
"author": "Riveiro",
"doi-asserted-by": "crossref",
"first-page": "1325",
"journal-title": "Curr. Med. Chem.",
"key": "ref_68",
"volume": "17",
"year": "2010"
},
{
"DOI": "10.1016/j.lfs.2021.119105",
"article-title": "Network pharmacology and molecular docking reveal the mechanism of Scopoletin against non-small cell lung cancer",
"author": "Yuan",
"doi-asserted-by": "crossref",
"first-page": "119105",
"journal-title": "Life Sci.",
"key": "ref_69",
"volume": "270",
"year": "2021"
},
{
"DOI": "10.1016/B978-0-12-800018-2.00021-2",
"doi-asserted-by": "crossref",
"key": "ref_70",
"unstructured": "Kuete, V. (2014). 20-Harmful and Protective Effects of Phenolic Compounds from African Medicinal Plants. Toxicological Survey of African Medicinal Plants, Elsevier."
},
{
"DOI": "10.3389/fmicb.2020.01525",
"doi-asserted-by": "crossref",
"key": "ref_71",
"unstructured": "Lemos, A.S.O., Florêncio, J.R., Pinto, N.C.C., Campos, L.M., Silva, T.P., Grazul, R.M., Pinto, P.F., Tavares, G.D., Scio, E., and Apolônio, A.C.M. (2020). Antifungal Activity of the Natural Coumarin Scopoletin Against Planktonic Cells and Biofilms From a Multidrug-Resistant Candida tropicalis Strain. Front. Microbiol., 11."
}
],
"reference-count": 71,
"references-count": 71,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.mdpi.com/1999-4915/17/3/402"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Biochemical Screening of Phytochemicals and Identification of Scopoletin as a Potential Inhibitor of SARS-CoV-2 Mpro, Revealing Its Biophysical Impact on Structural Stability",
"type": "journal-article",
"volume": "17"
}
