Feb 27 |
Lopinavir/ritonavir for COVID-19: real-time meta-analysis of 17 studies (Version 7) | |
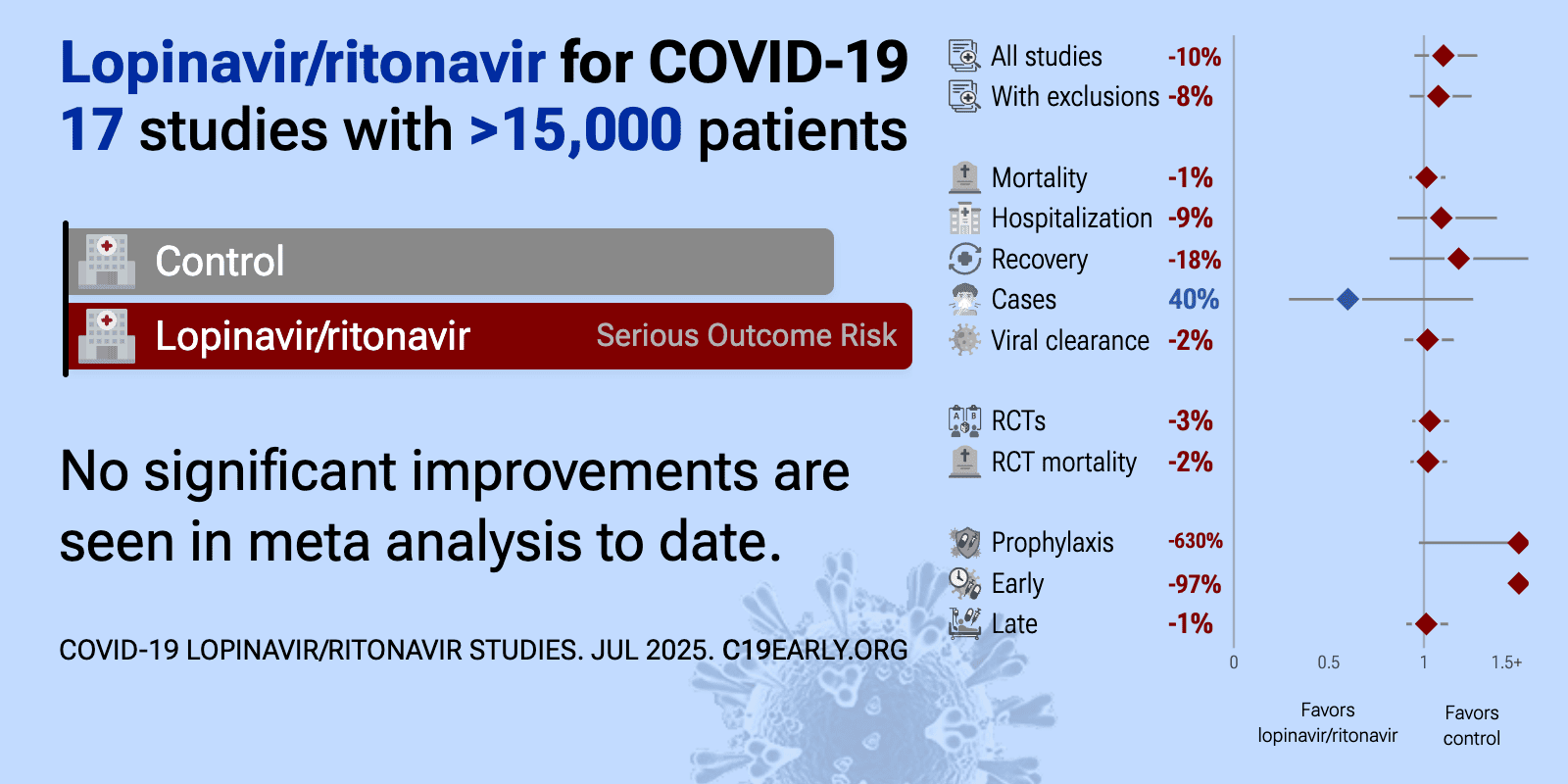
| Meta-analysis using the most serious outcome reported shows 11% [-4‑28%] higher risk, without reaching statistical significance. Control Lopinavir/ritonavirLopinavir/r.. All data and sources to reproduce this analysis are in th.. | ||
Sep 2 2025 |
et al., medRxiv, doi:10.1101/2025.08.29.25334732 | Long-term follow-up of treatment comparisons in RECOVERY: a randomised, open-label, platform trial for patients hospitalised with COVID-19 |
| 6-month followup of RECOVERY patients. Results are reported within the respective trials for each treatment. | ||
Aug 13 2024 |
et al., Cureus, doi:10.7759/cureus.66798 | Anticoagulant Use in COVID-19 Patients: A Longitudinal Study From Zanjan, Iran |
| 163% higher mortality (p<0.0001). Retrospective 831 hospitalized COVID-19 patients showing higher mortality with lopinavir/ritonavir treatment in unadjusted results. | ||
Jul 30 2024 |
et al., Journal of Controversies in Obstetrics & Gynecology and Pediatrics, doi:10.51271/JCOGP-0035 | Is vitamin D level important in pregnant women with COVID-19? |
| 136% higher hospitalization (p=0.43). Retrospective 125 pregnant women hospitalized with COVID-19 in Turkey, showing no significant difference in hospitalization length with HCQ, and longer hospitalization with lopinavir/ritonavir use. | ||
Mar 31 2023 |
et al., International Journal of Infectious Diseases, doi:10.1016/j.ijid.2022.12.028 | Lopinavir/ritonavir for treatment of non-hospitalized patients with COVID-19: a randomized clinical trial |
| 203% higher mortality (p=0.49), 20% higher hospitalization (p=0.78), and 3% worse recovery (p=0.88). RCT 437 non-hospitalized COVID-19 patients showing no significant differences with lopinavir/ritonavir (LPV/r) treatment. | ||
Apr 16 2022 |
et al., Pediatric Drugs, doi:10.1007/s40272-022-00500-7 | Slower Recovery with Early Lopinavir/Ritonavir use in Pediatric COVID-19 Patients: A Retrospective Observational Study |
| 96% lower hospital discharge (p<0.0001) and 96% worse recovery (p<0.0001). Retrospective 933 pediatric COVID-19 patients in Hong Kong showing worse outcomes with early lopinavir/ritonavir (LPV/r) use. | ||
Feb 15 2022 |
et al., PLOS Medicine, doi:10.1371/journal.pmed.1004120 (date from preprint) | Favipiravir, lopinavir-ritonavir, or combination therapy (FLARE): A randomised, double-blind, 2 × 2 factorial placebo-controlled trial of early antiviral therapy in COVID-19 |
| 200% higher hospitalization (p=1) and 7% improved viral clearance (p=0.56). 240 patient RCT comparing favipiravir, favipiravir + LPV/r, LPV/r, and placebo, showing improved viral clearance with favipiravir, but no significant difference for LPV/r. Efficacy was lower in the combined favipiravir + LPV/r arm, where .. | ||
Dec 31 2021 |
et al., eClinicalMedicine, doi:10.1016/j.eclinm.2021.101188 | Post-exposure Lopinavir-Ritonavir Prophylaxis versus Surveillance for Individuals Exposed to SARS-CoV-2: The COPEP Pragmatic Open-Label, Cluster Randomized Trial |
| 630% higher progression (p=0.02) and 40% fewer symptomatic cases (p=0.17). Open-label, cluster-randomized RCT 318 asymptomatic close contacts in Switzerland and Brazil showing no statistically significant difference in symptomatic COVID-19 at 21 days with LPV/r prophylaxis. The mid-trial changes in allocation an.. | ||
Jul 12 2021 |
et al., Intensive Care Medicine | Lopinavir-ritonavir and hydroxychloroquine for critically ill patients with COVID-19: REMAP-CAP randomized controlled trial |
| 54% higher mortality (p=0.02). Very late stage RCT with 50 ICU patients treated with HCQ, 255 lopinavir-ritonavir patients, and 27 combined therapy patients, showing higher mortality with all treatments. Authors do not report results for patients in the moderate state,.. | ||
Jun 9 2021 |
et al., Frontiers in Medicine, doi:10.3389/fmed.2021.639970 | Lopinavir/Ritonavir and Darunavir/Cobicistat in Hospitalized COVID-19 Patients: Findings From the Multicenter Italian CORIST Study |
| 6% lower mortality (p=0.52). Retrospective 3,451 hospitalized COVID-19 patients in Italy showing higher mortality with darunavir/cobicistat. | ||
Apr 28 2021 |
et al., Respiratory Medicine, doi:10.1016/j.rmed.2021.106433 | The predictors of COVID-19 mortality in a nationwide cohort of Turkish patients |
| 118% higher mortality (p=0.38). Retrospective 1,500 hospitalized late stage (median SaO2 87.7) patients in Turkey, showing no significant difference in mortality with treatment. | ||
Apr 22 2021 |
et al., JAMA Network Open, doi:10.1001/jamanetworkopen.2021.6468 | Effect of Early Treatment With Hydroxychloroquine or Lopinavir and Ritonavir on Risk of Hospitalization Among Patients With COVID-19 The TOGETHER Randomized Clinical Trial |
| 86% higher mortality (p=1) and 16% higher hospitalization (p=0.72). Early terminated RCT in Brazil showing no significant differences with lopinavir/ritonavir treatment. | ||
Dec 31 2020 |
et al., Med, doi:10.1016/j.medj.2020.04.001 | Efficacy and Safety of Lopinavir/Ritonavir or Arbidol in Adult Patients with Mild/Moderate COVID-19: An Exploratory Randomized Controlled Trial |
| 100% higher progression (p=0.46) and 3% faster viral clearance (p=0.84). RCT 86 mild/moderate COVID-19 patients showing no significant difference in outcomes with lopinavir/ritonavir or arbidol compared to control. | ||
Oct 31 2020 |
et al., The Lancet, doi:10.1016/S0140-6736(20)32013-4 | Lopinavir–ritonavir in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial |
| 7% higher mortality (p=0.24), 15% higher ventilation (p=0.15), and 2% lower hospital discharge (p=0.53). RCT 1,616 hospitalized COVID-19 patients showing no significant differences with lopinavir-ritonavir treatment compared to usual care. 6-month results are from [Horby] | ||
Oct 20 2020 |
et al., Biomedical Reports, doi:10.3892/br.2020.1375 | Retrospective analysis of the effect of current clinical medications and clinicopathological factors on viral shedding in COVID‑19 patients |
| 45% improved viral clearance (p=0.01). Retrospective 186 hospitalized COVID-19 patients showing faster viral clearance with lopinavir/ritonavir. There may be significant confounding by indication - authors classify cases as non-severe vs severe, but severity is not included in.. | ||
Oct 15 2020 |
, NEJM, doi:10.1056/NEJMoa2023184 (date from preprint) | Repurposed antiviral drugs for COVID-19; interim WHO SOLIDARITY trial results |
| no change in mortality (p=1) and 2% higher ventilation (p=0.89). WHO SOLIDARITY open-label RCT showing no significant difference in outcomes with lopinavir/ritonavir treatment. | ||
Oct 6 2020 |
et al., medRxiv, doi:10.1101/2022.02.16.22271064 | An open-label randomized, controlled trial of the effect of lopinavir/ritonavir, lopinavir/ritonavir plus IFN-beta-1a and hydroxychloroquine in hospitalized patients with COVID-19 - Final results from the DisCoVeRy trial |
| 36% higher mortality (p=0.39) and 26% improved viral clearance (p=0.65). Early terminated very late stage (95% on oxygen at baseline) DISCOVERY trial showing no significant differences with lopinavir/ritonavir. | ||
Aug 1 2020 |
et al., Zhonghua Nei Ke Za Zhi, doi:10.3760/cma.j.cn112138-20200227-00147 | The efficacy and safety of lopinavir/ritonavir and arbidol in patients with coronavirus disease 2019 |
| 21% slower viral clearance (p=0.008). Retrospective 178 hospitalized COVID-19 patients in China | ||
Jul 14 2020 |
et al., Frontiers in Pharmacology, doi:10.3389/fphar.2020.01071 | No Statistically Apparent Difference in Antiviral Effectiveness Observed Among Ribavirin Plus Interferon-Alpha, Lopinavir/Ritonavir Plus Interferon-Alpha, and Ribavirin Plus Lopinavir/Ritonavir Plus Interferon-Alpha in Patients With Mild to Moderate Coronavirus Disease 2019: Results of a Randomized, Open-Labeled Prospective Study |
| 6% longer hospitalization (p=0.68), 33% faster recovery (p=0.32), 18% faster improvement (p=0.07), and 15% slower viral clearance (p=0.41). RCT 101 mild to moderate COVID-19 patients showing no significant difference in antiviral effectiveness among three treatment regimens: ribavirin plus interferon-alpha, lopinavir/ritonavir plus interferon-alpha, and ribavirin plus lopinav.. | ||
May 19 2020 |
et al., European Respiratory Journal, doi:10.1183/13993003.00799-2020 | Factors associated with prolonged viral shedding and impact of lopinavir/ritonavir treatment in hospitalised non-critically ill patients with SARS-CoV-2 infection |
| 40% improved viral clearance (p=0.01). Retrospective 120 hospitalized non-critically ill COVID-19 patients showing that early administration of lopinavir/ritonavir was associated with shorter duration of SARS-CoV-2 RNA shedding. | ||
May 7 2020 |
et al., New England Journal of Medicine, doi:10.1056/NEJMoa2001282 | A Trial of Lopinavir–Ritonavir in Adults Hospitalized with Severe Covid-19 |
| 23% lower mortality (p=0.39), 29% greater improvement (p=0.19), and 4% improved viral clearance (p=1). RCT 199 hospitalized COVID-19 patients showing no significant difference with lopinavir-ritonavir treatment. 28-day mortality was lower in the treatment group, without statistical significance. 3 treatment patients died within 24 hours af.. | ||
Mar 31 2020 |
et al., The Lancet, doi:10.1016/S0140-6736(20)30566-3 | Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study |
| 10% slower viral clearance (p=0.06). Retrospective 191 hospitalized COVID-19 patients in China showing no significant difference in viral clearance with lopinavir/ritonavir. | ||