Recent:Park.
Feb 27 |
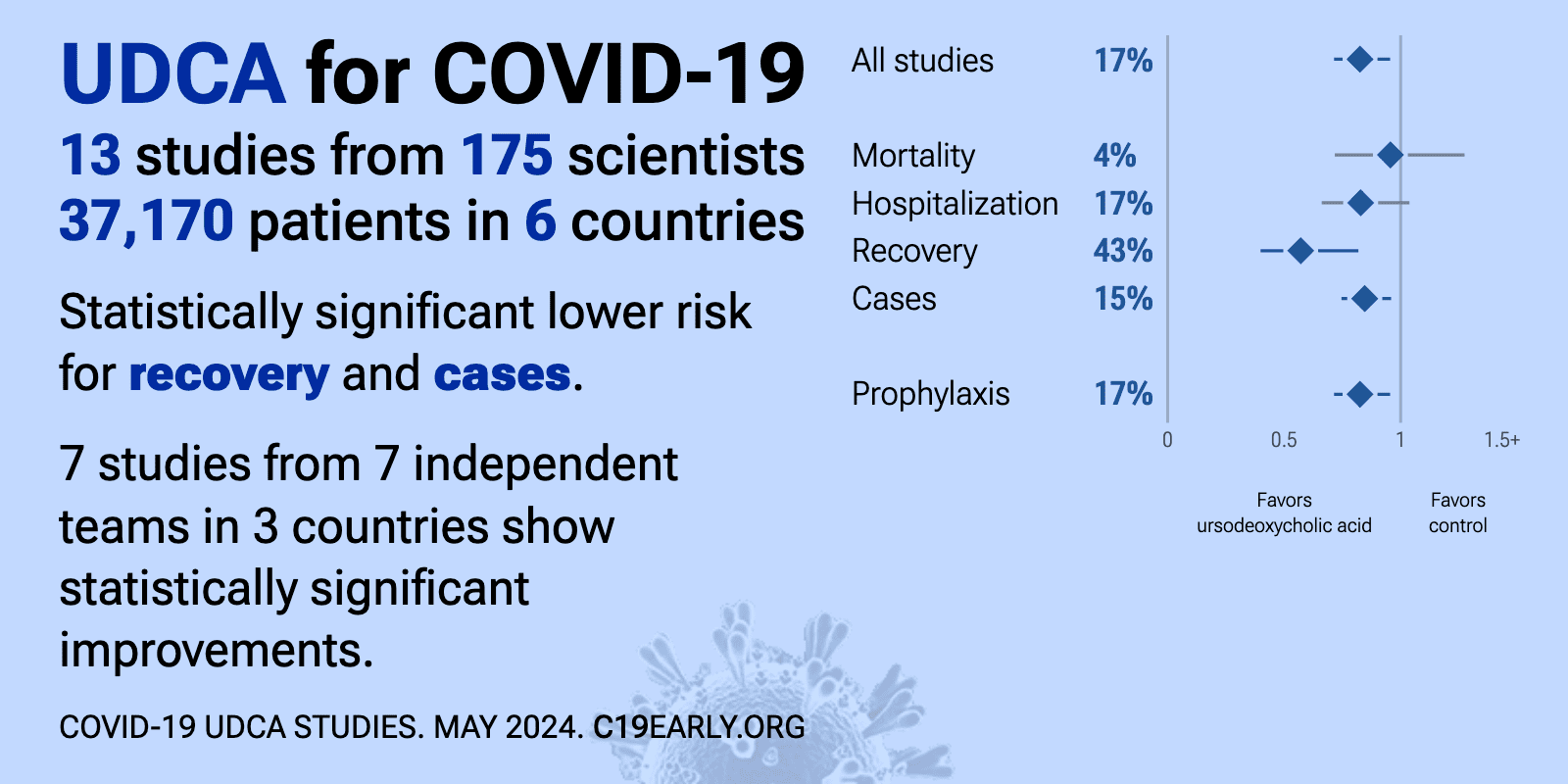
Ursodeoxycholic acid for COVID-19: real-time meta-analysis of 21 studies (Version 19) | |
| Significantly lower risk is seen for progression, recovery, and cases. 13 studies from 12 independent teams in 4 countries show significant benefit. Meta-analysis using the most serious outcome reported shows 19% [-3‑36%] lower.. | ||
Oct 17 2025 |
et al., Biomolecules & Therapeutics, doi:10.4062/biomolther.2025.149 | Ursodeoxycholic Acid Attenuates B Cell Susceptibility to SARS-CoV-2 Spike Protein by Interfering Its Binding to ACE2 |
| In vitro study showing that ursodeoxycholic acid (UDCA) reduces SARS-CoV-2 spike protein binding to ACE2 and protects B cells from enhanced susceptibility caused by bisphenol A (BPA) exposure. | ||
Feb 6 2025 |
et al., Frontiers in Medicine, doi:10.3389/fmed.2025.1494248 | Ursodeoxycholic acid relieves clinical severity of COVID-19 in patients with chronic liver diseases |
| 89% lower severe cases (p=0.02) and 25% fewer symptomatic cases (p<0.0001). Retrospective 926 outpatients with chronic liver diseases in China showing lower incidence of symptomatic COVID-19 and milder symptoms with ursodeoxycholic acid (UDCA) treatment. | ||
Feb 1 2025 |
et al., Internal Medicine, doi:10.2169/internalmedicine.4856-24 | Use of Ursodeoxycholic Acid and the Risk of Severe Coronavirus Disease 2019 in Elderly Patients with Viral Hepatitis |
| 72% higher mortality (p=0.03), 57% higher ventilation (p=0.48), 3% higher need for oxygen therapy (p=0.83), and 4% higher hospitalization (p=0.52). Retrospective 6,413 elderly patients with viral hepatitis in Japan showing increased mortality with ursodeoxycholic acid (UDCA) use in COVID-19 patients. There was no significant difference in hospitalization or oxygen therapy. | ||
Nov 20 2024 |
et al., Research Square, doi:10.21203/rs.3.rs-5317838/v1 | Ursodeoxycholic acid reduces ACE-2 activity in COVID-19 patients and Calu- 3 cells |
| Retrospective 142 COVID-19 patients (89 treated with UDCA, 53 UDCA-free) showing reduced ACE2 levels in serum, plasma, and blood cells, a shorter time to fever resolution, and no significant difference in respiratory improvement with UDCA.. | ||
Sep 27 2024 |
et al., Discover Public Health, doi:10.1186/s12982-024-00225-7 | Insights from a multicenter nationwide cohort analysis in Japan on the association of underlying conditions and pharmacological interventions with COVID-19 disease severity |
| 79% higher severe cases (p<0.0001). Retrospective 650,317 COVID-19 patients in Japan showing higher risk of severe COVID-19 with UDCA use. There may be significant residual confounding because authors do not appear to have adjusted for liver diseases (details of adjustments.. | ||
Aug 27 2024 |
et al., Virology Journal, doi:10.1186/s12985-024-02464-1 | Association between ursodeoxycholic acid use and COVID-19 in individuals with chronic liver disease: a nationwide case-control study in South Korea |
| 33% lower severe cases (p=0.04) and 20% fewer cases (p<0.0001). Retrospective 74,074 individuals with chronic liver disease in South Korea, showing lower risk of COVID-19 infection and related severe outcomes with ursodeoxycholic acid (UDCA) use. The risk reduction was dose-dependent, with greater ben.. | ||
Aug 14 2024 |
et al., JMIR Public Health and Surveillance, doi:10.2196/59274 | Association Between Ursodeoxycholic Acid and Clinical Outcomes in Patients With COVID-19 Infection: Population-Based Cohort Study |
| Retrospective 1,675,593 patients in the Jeonbuk CDM cohort and 8,528,533 patients in the NHIS cohort, showing ursodeoxycholic acid (UDCA) intake associated with significantly lower risk of COVID-19 infection and severe COVID-19. | ||
Jul 31 2024 |
et al., Frontiers in Pharmacology, doi:10.3389/fphar.2024.1381830 | Evaluating the protective effectiveness and risk factors of ursodeoxycholic acid on COVID-19 among outpatients |
| 21% fewer symptomatic cases (p=0.001), 19% fewer cases (p=0.004), and 18% lower progression (p=0.04). Retrospective 1,040 outpatients in China showing lower COVID-19 cases, less severe symptoms, and shorter symptom duration with ursodeoxycholic acid (UDCA) use. | ||
Jul 25 2024 |
et al., Journal of Clinical Hepatology, doi:10.12449/JCH240714 | Effect of ursodeoxycholic acid on symptoms after severe acute respiratory syndrome coronavirus 2 infection in patients with primary biliary cholangitis and their family members |
| Retrospective 171 primary biliary cholangitis (PBC) patients taking ursodeoxycholic acid (UDCA) and 128 family members, showing no reduction in SARS-CoV-2 infection rates but milder symptoms in PBC patients. All PBC patients and family me.. | ||
Jul 8 2024 |
et al., Expert Review of Anti-infective Therapy, doi:10.1080/14787210.2024.2376153 | Ursodeoxycholic acid and COVID-19 outcomes: a cohort study and data synthesis of state-of-art evidence |
| 3% lower mortality (p=0.5) and 32% lower severe cases (p=0.02). Meta analysis of 9 studies showing lower risk of severe/critical COVID-19 with UDCA. There was no significant difference for mortality. Authors also perform a retrospective study which is listed separately [Yu]. | ||
Jul 8 2024 |
et al., Expert Review of Anti-infective Therapy, doi:10.1080/14787210.2024.2376153 | Ursodeoxycholic acid and COVID-19 outcomes: a cohort study and data synthesis of state-of-art evidence |
| 39% faster recovery (p=0.04). Retrospective 115 hospitalized COVID-19 patients in China showing faster time to body temperature recovery with ursodeoxycholic acid (UDCA) treatment. Results were better for higher dose treatment (300mg vs. 150mg). Authors also perform a.. | ||
Jun 22 2024 |
et al., Microorganisms, doi:10.3390/microorganisms12071269 | The Value of Ursodeoxycholic Acid and Mesenchymal Stem Cells in the Treatment of Severe COVID-19 |
| 62% lower mortality (p=0.03). Retrospective 167 severe COVID-19 patients showing lower mortality with ursodeoxycholic acid (UDCA). Timing and duration of treatment is unknown - UDCA patients may have been on UDCA since before COVID-19. | ||
Apr 4 2024 |
et al., Pharmacology Research & Perspectives, doi:10.1002/prp2.1194 | Ursodeoxycholic acid may protect from severe acute respiratory syndrome coronavirus 2 Omicron variant by reducing angiotensin‐converting enzyme 2 |
| Syrian hamster study showing ursodeoxycholic acid (UDCA) may protect against SARS-CoV-2 omicron variant transmission and infection. Hamsters treated prophylactically with oral UDCA had significantly less weight loss compared to untreated .. | ||
Mar 20 2024 |
et al., Journal of Clinical Hepatology, doi:10.12449/JCH240309 | Efficacy of ursodeoxycholic acid in the prevention and treatment of COVID-19 in patients with chronic hepatitis B |
| 48% faster recovery (p=0.01) and 83% fewer cases (p=0.001). Retrospective 215 patients with chronic hepatitis B in China, showing lower risk of COVID-19 infection, milder symptoms, and faster recovery with ursodeoxycholic acid (UDCA) treatment. | ||
Feb 13 2024 |
et al., Acta Pharmaceutica Sinica B, doi:10.1016/j.apsb.2024.02.011 | Bile acids and coronavirus disease 2019 |
| Review of the relationship between bile acids and COVID-19. Authors discuss emerging basic and clinical evidence linking bile acids to COVID-19 infection and potential mechanisms. Functionally, studies indicate that certain bile acids lik.. | ||
Jan 19 2024 |
et al., Journal of Medical Virology, doi:10.1002/jmv.29418 | Exploring the impact of ursodeoxycholic acid therapy on COVID‐19 in a real‐world setting |
| 19% lower ICU admission (p=1) and 40% lower hospitalization (p=0.17). Retrospective cohort study of 10,147 chronic liver disease patients in France, with 1,322 exposed to ursodeoxycholic acid (UDCA), showing lower risk of hospitalization for COVID-19 with UDCA exposure, without statistical significance (adj.. | ||
Dec 13 2023 |
et al., Communications Medicine, doi:10.1038/s43856-024-00664-y (date from preprint) | Ursodeoxycholic acid and severe COVID-19 outcomes in a cohort study using the OpenSAFELY platform |
| 24% lower mortality (p=0.13), 19% lower hospitalization (p=0.02), and 21% lower combined mortality/hospitalization (p=0.005). OpenSAFELY retrospective 11,305 primary biliary cholangitis (PBC) and primary sclerosing cholangitis (PSC) patients showing lower risk of COVID-19 hospitalization or death with ursodeoxycholic acid (UDCA) treatment. | ||
Dec 9 2023 |
et al., Biochemical Pharmacology, doi:10.1016/j.bcp.2023.115983 | Bile acids and bile acid activated receptors in the treatment of Covid-19 |
| Review of the potential role of bile acids and bile acid activated receptors in modulating SARS-CoV-2 infectivity and inflammation in COVID-19. Authors discuss mechanisms by which bile acids like ursodeoxycholic acid (UDCA), chenodeoxycho.. | ||
Nov 28 2023 |
et al., Blood, doi:10.1182/blood-2023-182862 | Effect of Ursodeoxycholic Acid for Sars-Cov-2 Prevention in Hematological Malignancies: An Observational Real-World Study |
| 12% fewer cases (p=0.03). Retrospective 393 hospitalized patients with hematologic disorders in China, showing lower risk of COVID-19 with UDCA use. | ||
Nov 16 2023 |
et al., Microbiology and Infectious Diseases The American Medical Journal, doi:10.33590/microbiolinfectdisamj/10304488 | A Retrospective Study in Patients With Varying Prescription Coverage With Ursodeoxycholic Acid and Association With Incidence of COVID-19 Diagnosis in Primary Care |
| 13% fewer cases (p=0.03). Retrospective 8,964 primary care patients prescribed ursodeoxycholic acid (UDCA) in the UK. Higher categorized UDCA adherence (≥80%) was associated with lower COVID-19 incidence (OR 0.86), whereas adherence as a continuous variable was no.. | ||
Sep 21 2023 |
et al., Liver International, doi:10.1111/liv.15736 | Ursodeoxycholic acid does not affect the clinical outcome of SARS‐CoV‐2 infection: A retrospective study of propensity score‐matched cohorts |
| 7% higher mortality (p=0.77). PSM retrospective 629 hospitalized COVID-19 patients showing no significant difference in survival between 108 patients taking UDCA prior to infection compared to 521 matched controls not taking the drug. The lack of observed benefit in t.. | ||
Aug 22 2023 |
et al., Journal of Internal Medicine, doi:10.1111/joim.13711 | UDCA treatment against COVID‐19: Do we have enough clinical evidence for drug repurposing? |
| 7% higher mortality (p=0.67), 4% lower ICU admission (p=0.96), 6% higher hospitalization (p=0.66), and 3% fewer cases (p=0.77). Retrospective cohort study of 9,617 patients with liver disease in Italy, divided into UDCA users and non-users. UDCA exposure was not associated with reduced SARS-CoV-2 infection or improved COVID-19 outcomes including death, hospitaliza.. | ||
Aug 14 2023 |
et al., Viruses, doi:10.3390/v15081738 | Ursodeoxycholic Acid Does Not Improve COVID-19 Outcome in Hospitalized Patients |
| no change in mortality (p=0.24). Retrospective 3,847 COVID-19 patients hospitalized in Italy, including 57 treated with UDCA. UDCA treatment was not associated with reduced mortality, however treatment was associated with a lower rate of CPAP use. It's not clear how the .. | ||
Jul 27 2023 |
et al., Journal of Internal Medicine, doi:10.1111/joim.13704 | Ursodeoxycholic acid for coronavirus disease 2019 prevention |
| 17% fewer symptomatic cases (p=0.81) and no change in cases (p=1). Retrospective 94 outpatients attending a university hospital gastroenterology clinic in Japan showing no significant difference in SARS-CoV-2 infection rates between ursodeoxycholic acid (UDCA)-treated patients and control groups without .. | ||
Jun 29 2023 |
et al., Liver International, doi:10.1111/liv.15660 | Ursodeoxycholic acid administration did not reduce susceptibility to SARS‐CoV‐2 infection in children |
| 2% more cases (p=0.88). Retrospective 280 Chinese families with children previously seen in a liver clinic assessing whether ursodeoxycholic acid (UDCA) reduced SARS-CoV-2 infection risk. Among infected families, the study found no significant difference in conf.. | ||
May 4 2023 |
et al., medRxiv, doi:10.1101/2023.05.02.23289410 | UDCA May Promote COVID-19 Recovery: A Cohort Study with AI-Aided Analysis |
| 38% improved recovery (p=0.05). Retrospective 115 COVID-19 patients hospitalized during an Omicron outbreak in China, of which 65 received ursodeoxycholic acid (UDCA) treatment and 50 received standard care. It found that UDCA was associated with faster body temperature.. | ||
May 3 2023 |
et al., Frontiers in Cellular and Infection Microbiology, doi:10.3389/fcimb.2023.1178590 | Protective effect of ursodeoxycholic acid on COVID-19 in patients with chronic liver disease |
| 80% lower severe cases (p=0.22), 80% fewer moderate/severe cases (p<0.0001), and 11% fewer cases (p=0.05). Retrospective propensity score matched cohort study of 225 chronic liver disease patients on UDCA therapy matched to 225 controls without UDCA in China. UDCA use was associated with lower COVID-19 infection rate (85% vs 94%), lower maximu.. | ||
Apr 5 2023 |
et al., Journal of Internal Medicine, doi:10.1111/joim.13630 | Ursodeoxycholic acid is associated with a reduction in SARS‐CoV‐2 infection and reduced severity of COVID‐19 in patients with cirrhosis |
| 42% lower mortality (p=0.28), 54% lower severe cases (p=0.03), 55% fewer moderate/severe cases (p=0.002), and 50% fewer symptomatic cases (p<0.0001). Retrospective 3,214 veterans with cirrhosis comparing 1,607 participants taking ursodeoxycholic acid (UDCA) to 1,607 propensity score matched controls not taking UDCA. UDCA use was associated with significantly lower odds of SARS-CoV-2 in.. | ||
Dec 5 2022 |
et al., Nature, doi:10.1038/s41586-022-05594-0 | FXR inhibition may protect from SARS-CoV-2 infection by reducing ACE2 |
| 94% lower mortality (p=0.13), 75% lower ICU admission (p=0.21), and 40% lower hospitalization (p=0.03). Retrospective study from two registries of 1,096 COVID-19 patients with chronic liver disease, including 31 treated with ursodeoxycholic acid (UDCA). Propensity score matching was used to compare outcomes between UDCA-treated and untreate.. | ||