Feb 19 |
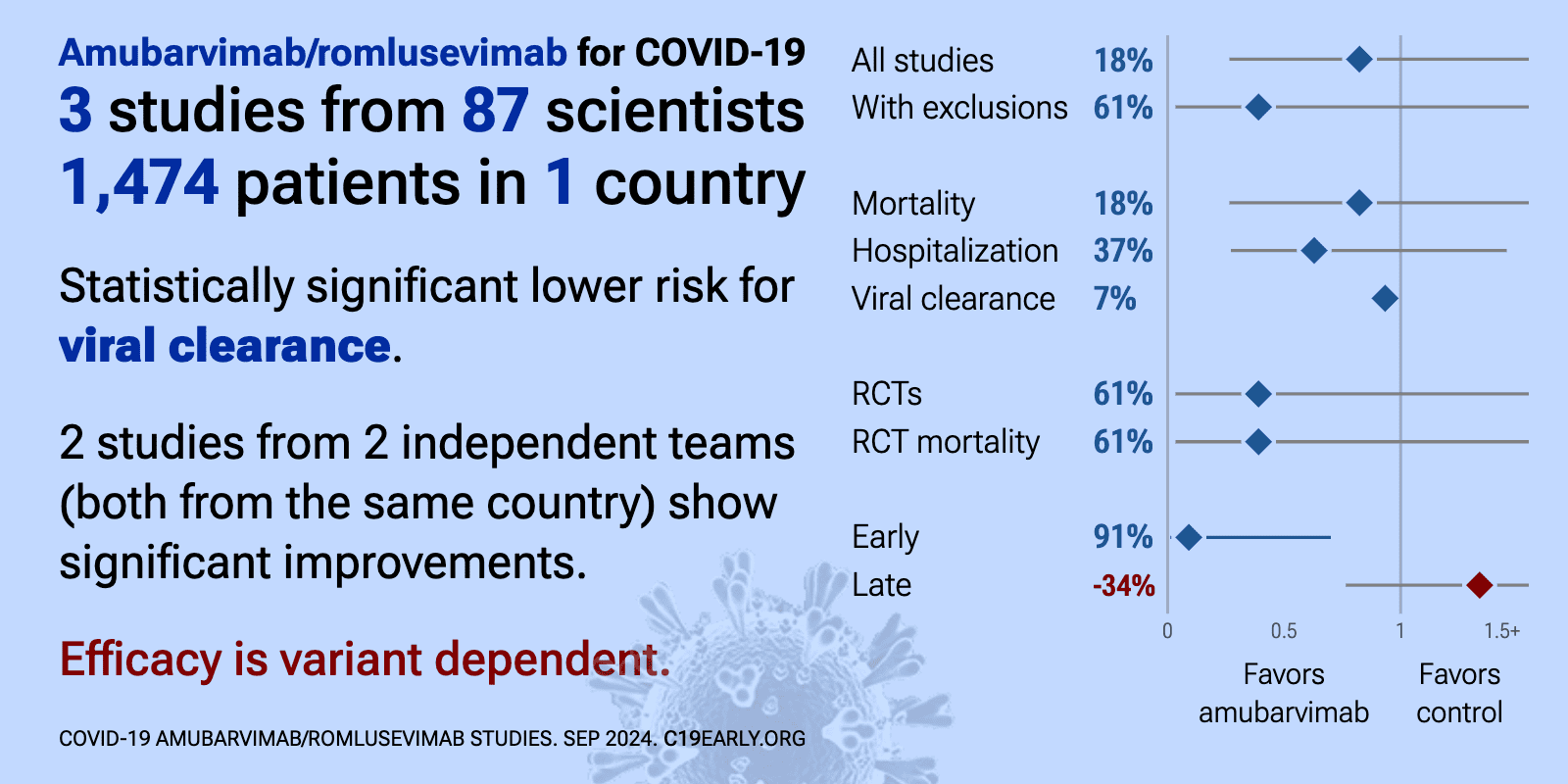
Amubarvimab/romlusevimab for COVID-19: real-time meta-analysis of 4 studies (Version 4) | |
| Significantly lower risk is seen for viral clearance. 2 studies from 2 independent teams (both from the same country) show significant benefit. Meta-analysis using the most serious outcome reported shows 25% [-70‑66%] lower ris.. | ||
Feb 6 2025 |
et al., Clinical Microbiology and Infection, doi:10.1016/j.cmi.2025.02.002 | Long-term outcomes of passive immunotherapy for COVID-19: a pooled analysis of a large multinational platform randomized clinical trial |
| 54% higher mortality (p=0.19) and 12% higher combined mortality/hospitalization (p=0.53). Analysis of 2,311 hospitalized COVID-19 patients from four randomized controlled trials (ACTIV-3/TICO) showing no significant difference in long-term mortality with sotrovimab, amubarvimab/romlusevimab, tixagevimab-cilgavimab, or ensovibep. | ||
Sep 30 2024 |
et al., Heliyon, doi:10.1016/j.heliyon.2024.e37663 | Effect of amubarvimab-romlusevimab for treatment of severe COVID-19 in intensive care units: A retrospective cohort study |
| 46% lower mortality (p=0.06) and 4% improved viral clearance (p=0.89). Retrospective 121 severe ICU COVID-19 patients in China showing lower 28-day mortality and ICU mortality with amubarvimab-romlusevimab treatment compared to no antiviral treatment. No significant differences were found in viral conversion.. | ||
Aug 16 2024 |
et al., eClinicalMedicine, doi:10.1016/j.eclinm.2024.102787 | Post-acute COVID-19 outcomes including participant-reported long COVID: amubarvimab/romlusevimab versus placebo in the ACTIV-2 trial |
| 91% lower mortality (p=0.006), 20% higher long COVID (p=0.39), 61% lower hospitalization (p=0.0008), and 67% lower combined mortality/hospitalization (p<0.0001). RCT 780 high-risk non-hospitalized COVID-19 patients showing significantly lower risk of hospitalization or death through 36 weeks, but no significant difference in long COVID with amubarvimab/romlusevimab treatment compared to placebo. | ||
Aug 11 2024 |
, D., Current Topics in Microbiology and Immunology, doi:10.1007/82_2024_268 | Monoclonal Antibody Therapies Against SARS-CoV-2: Promises and Realities |
| Review of monoclonal antibodies for SARS-CoV-2. Author notes that the omicron variant has reset achievements to date. | ||
Aug 8 2024 |
et al., Clinical Infectious Diseases, doi:10.1093/cid/ciae408 | Single monoclonal antibodies should not be used for COVID-19 therapy: a call for antiviral stewardship |
| Review arguing against use of single monoclonal antibodies for COVID-19 treatment, particularly in immunosuppressed patients, due to the risk of rapidly selecting for resistant viral variants. Authors suggest that while monoclonal antibod.. | ||
Apr 19 2024 |
et al., BMC Pharmacology and Toxicology, doi:10.1186/s40360-024-00753-7 | Treatment for Covid-19 with SARS-CoV-2 neutralizing antibody BRII-196(Ambavirumab) plus BRII-198(Lomisivir): a retrospective cohort study |
| 71% higher mortality (p=0.35), 8% higher ICU admission (p=0.8), 8% shorter hospitalization (p=0.004), and 7% faster viral clearance (p=0.004). Retrospective 340 COVID-19 patients in China showing shorter length of hospital stay and faster viral clearance with BRII-196 plus BRII-198 monoclonal antibody treatment, especially when given early. The treatment did not show efficacy fo.. | ||
Sep 6 2022 |
et al., Frontiers in Pharmacology, doi:10.3389/fphar.2022.983505 | Randomized, placebo-controlled, single-blind phase 1 studies of the safety, tolerability, and pharmacokinetics of BRII-196 and BRII-198, SARS-CoV-2 spike-targeting monoclonal antibodies with an extended half-life in healthy adults |
| Phase 1 RCT with 44 healthy adults showing BRII-196 and BRII-198 monoclonal antibodies, alone or in combination, were safe and well-tolerated up to the highest doses tested of 3,000 mg for single mAbs and 1500/1500 mg for the combination... | ||
Dec 23 2021 |
et al., The Lancet Infectious Diseases, doi:10.1016/S1473-3099(21)00751-9 | Efficacy and safety of two neutralising monoclonal antibody therapies, sotrovimab and BRII-196 plus BRII-198, for adults hospitalised with COVID-19 (TICO): a randomised controlled trial |
| 15% higher mortality (p=0.72) and 7% improved recovery (p=0.48). RCT with 182 sotrovimab patients, 176 BRII-196+BRII-198 patients, and 178 control patients, median 8 days from symptom onset, showing no significant differences and terminated early due to futility. Long-term results are reported in [Mou.. | ||
References
Focosi et al., Analysis of SARS-CoV-2 mutations associated with resistance to therapeutic monoclonal antibodies that emerge after treatment, Drug Resistance Updates, doi:10.1016/j.drup.2023.100991.
Leducq et al., Spike protein genetic evolution in patients at high-risk of severe COVID-19 treated by monoclonal antibodies, The Journal of Infectious Diseases, doi:10.1093/infdis/jiad523.
Bruhn et al., Somatic hypermutation shapes the viral escape profile of SARS-CoV-2 neutralising antibodies, eBioMedicine, doi:10.1016/j.ebiom.2025.105770.
Ngiam et al., Early administration of neutralising monoclonal antibodies and post-acute sequelae of COVID-19, International Journal of Infectious Diseases, doi:10.1016/j.ijid.2026.108435.
Choudhary et al., Emergence of SARS-CoV-2 Resistance with Monoclonal Antibody Therapy, medRxiv, doi:10.1101/2021.09.03.21263105.
Günther et al., Variant-specific humoral immune response to SARS-CoV-2 escape mutants arising in clinically severe, prolonged infection, medRxiv, doi:10.1101/2024.01.06.24300890.
Casadevall et al., Single monoclonal antibodies should not be used for COVID-19 therapy: a call for antiviral stewardship, Clinical Infectious Diseases, doi:10.1093/cid/ciae408.