Efficacy is variant dependent. In Vitro research shows reduced efficacy against KP.3.1.1, KP.1.1, LB.1, KP.3.3, and XEC variants1-4. mAb use may create new variants that spread globally5-7, and may be associated with increased risk of autoimmune disease8, prolonged viral loads, clinical deterioration, and immune escape6,9-13 .
Pemivibart was adopted
in 1 country.
May 26 2025 |
et al., Infectious Diseases, doi:10.1080/23744235.2025.2509011 | Dynamics of SARS-CoV-2 variants and mutations in Central Sweden between 2023 and 2024 and their potential implications on monoclonal antibodies pemivibart and sipavibart as PrEP in the region |
| Analysis of SARS-CoV-2 variants and mutations in central Sweden from October 2023 to October 2024, showing the rise of resistance mutations that likely render monoclonal antibodies sipavibart and pemivibart ineffective. | ||
Apr 8 2025 |
et al., Cell Reports, doi:10.1016/j.celrep.2025.115543 | Antibody evasiveness of SARS-CoV-2 subvariants KP.3.1.1 and XEC |
| In vitro study showing that VYD222 (pemivibart) shows reduced efficacy against emerging SARS-CoV-2 variants KP.3.1.1 and XEC due to mutations that affect RBD conformation and antibody binding. | ||
Jan 29 2025 |
et al., Open Forum Infectious Diseases, doi:10.1093/ofid/ofae631.2185 | Results from a Phase 1 First in Human Study of Pemivibart: An Extended Half-Life Monoclonal Antibody (mAb) |
| Phase 1 RCT with 30 healthy participants showing pemivibart was well-tolerated at doses up to 4500 mg, with no serious adverse events or adverse events leading to drug discontinuation reported. Pemivibart demonstrated linear and dose-prop.. | ||
Jan 29 2025 |
et al., Open Forum Infectious Diseases, doi:10.1093/ofid/ofae631.2191 | Pharmacokinetics (PK) and Serum Virus Neutralizing Antibody (sVNA) Titers Following the 2nd dose of Pemivibart in the Phase 3 CANOPY Trial |
| Pharmacokinetics (PK) and Serum Virus Neutralizing Antibody (sVNA) analysis for the CANOPY trial showing a second dose boosted serum virus neutralizing antibody titers by 17% compared to the first dose. A population pharmacokinetic model .. | ||
Nov 14 2024 |
et al., New England Journal of Medicine, doi:10.1056/NEJMc2404555 | Immunobridging for Pemivibart, a Monoclonal Antibody for Prevention of Covid-19 |
| Discussion of immunobridging data for the monoclonal antibody pemivibart for prevention of COVID-19 in immunocompromised individuals. Pemivibart received Emergency Use Authorization from the FDA based on safety and immunobridging data fro.. | ||
Nov 14 2024 |
et al., New England Journal of Medicine, doi:10.1056/NEJMc2410203 | Activity of Research-Grade Pemivibart against Recent SARS-CoV-2 JN.1 Sublineages |
| In vitro study showing that a laboratory-synthesized version of the monoclonal antibody pemivibart had reduced neutralization activity against recent SARS-CoV-2 JN.1 sublineages. Pemivibart was authorized for COVID-19 pre-exposure prophyl.. | ||
Nov 13 2024 |
et al., Clinical Infectious Diseases, doi:10.1093/cid/ciaf265 (date from preprint) | Safety and Efficacy of Pemivibart, a Long-Acting Monoclonal Antibody, for Prevention of Symptomatic COVID-19: Interim Results From a Phase 3 Randomized Clinical Trial (CANOPY) |
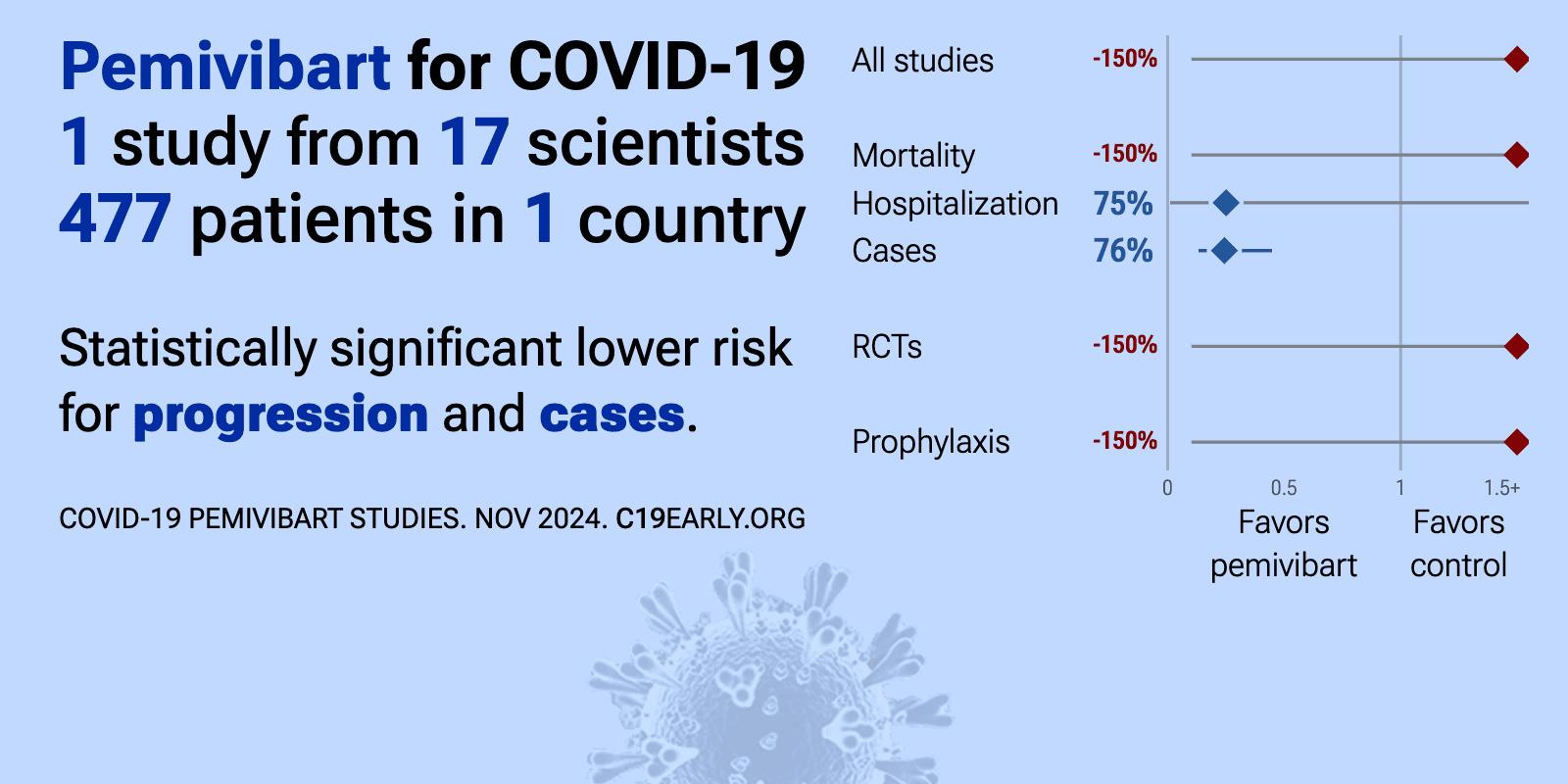
| 75% lower hospitalization (p=0.34), 74% lower progression (p<0.0001), and 76% fewer symptomatic cases (p<0.0001). Phase 3 trial of 306 immunocompromised adults and 484 non-immunocompromised adults showing pre-exposure prophylaxis with pemivibart was generally well-tolerated and provided protection against symptomatic COVID-19 through 6 months in.. | ||
Nov 13 2024 |
et al., bioRxiv, doi:10.1101/2024.11.11.623127 | Neutralization of recent SARS-CoV-2 variants by genetically and structurally related mAbs of the pemivibart lineage |
| In vitro study showing continued activity of pemivibart and 15 pemivibart-like monoclonal antibodies against recent SARS-CoV-2 variants KP.3, KP.3.1.1, and XEC. Authors found that all 15 antibodies maintained activity against KP.3.1.1, wi.. | ||
Nov 10 2024 |
et al., bioRxiv, doi:10.1101/2024.11.08.622746 | Neutralizing Activity and Viral Escape of Pemivibart by SARS-CoV-2 JN.1 sublineages |
| In vitro study showing that the monoclonal antibody pemivibart retains broad neutralizing activity against recent SARS-CoV-2 JN.1 sublineages but has reduced potency against KP.3.1.1 and XEC variants, with IC50 values ~22-fold higher than.. | ||
Oct 29 2024 |
et al., Clinical Infectious Diseases, doi:10.1093/cid/ciae435 | 2024 Clinical Practice Guideline Update by the Infectious Diseases Society of America on the Management of COVID-19: Anti-SARS-CoV-2 Neutralizing Antibody Pemivibart for Pre-exposure Prophylaxis |
| Update to IDSA clinical practice guidelines on the treatment and management of COVID-19, providing a conditional recommendation for pre-exposure prophylaxis with pemivibart, an anti-SARS-CoV-2 neutralizing antibody, in moderately or sever.. | ||
Sep 30 2024 |
et al., Pathogens and Immunity, doi:10.20411/pai.v10i1.752 | Escape of SARS-CoV-2 Variants KP.1.1, LB.1, and KP.3.3 From Approved Monoclonal Antibodies |
| In vitro study showing significant escape of SARS-CoV-2 variants KP.1.1, LB.1, and KP.3.3 with monoclonal antibodies pemivibart (VYD222) and sipavibart (AZD3152). Sipavibart lost antiviral efficacy, while pemivibart maintained reduced act.. | ||
Aug 11 2024 |
, D., Current Topics in Microbiology and Immunology, doi:10.1007/82_2024_268 | Monoclonal Antibody Therapies Against SARS-CoV-2: Promises and Realities |
| Review of monoclonal antibodies for SARS-CoV-2. Author notes that the omicron variant has reset achievements to date. | ||
Aug 8 2024 |
et al., Clinical Infectious Diseases, doi:10.1093/cid/ciae408 | Single monoclonal antibodies should not be used for COVID-19 therapy: a call for antiviral stewardship |
| Review arguing against use of single monoclonal antibodies for COVID-19 treatment, particularly in immunosuppressed patients, due to the risk of rapidly selecting for resistant viral variants. Authors suggest that while monoclonal antibod.. | ||
Mar 22 2024 |
, Press Release, 3/22 | Invivyd announces FDA authorization for emergency use of Pemgarda™ (formerly VYD222) for pre-exposure prophylaxis (PrEP) of COVID-19 |
| RCT 623 patients reporting immunobridging results from cohort A with 306 immunocompromised patients. Immunobridging estimates efficacy from the relationship between serum virus neutralizing antibody titers and clinical efficacy demonstrat.. | ||
Nov 27 2023 |
et al., Open Forum Infectious Diseases, doi:10.1093/ofid/ofad500.1200 | Preliminary Safety Results from a Phase 1 First in Human Study of VYD222: an Extended Half-Life Monoclonal Antibody (mAb) in Development for COVID-19 Prevention |
| Phase 1 RCT of monoclonal antibody VYD222 (pemivibart) in 12 healthy adults, showing no serious adverse events or infusion-related reactions through 14 days with 1500mg and through 2 days with 2500mg. | ||
References
Xie et al., Molecular Basis of High-Blood-Pressure-Enhanced and High-Fever-Temperature-Weakened Receptor-Binding Domain/Peptidase Domain Binding: A Molecular Dynamics Simulation Study, International Journal of Molecular Sciences, doi:10.3390/ijms26073250.
Wang et al., Activity of Research-Grade Pemivibart against Recent SARS-CoV-2 JN.1 Sublineages, New England Journal of Medicine, doi:10.1056/NEJMc2410203.
Yao et al., Neutralizing Activity and Viral Escape of Pemivibart by SARS-CoV-2 JN.1 sublineages, bioRxiv, doi:10.1101/2024.11.08.622746.
Planas et al., Escape of SARS-CoV-2 Variants KP.1.1, LB.1, and KP.3.3 From Approved Monoclonal Antibodies, Pathogens and Immunity, doi:10.20411/pai.v10i1.752.
Focosi et al., Analysis of SARS-CoV-2 mutations associated with resistance to therapeutic monoclonal antibodies that emerge after treatment, Drug Resistance Updates, doi:10.1016/j.drup.2023.100991.
Leducq et al., Spike protein genetic evolution in patients at high-risk of severe COVID-19 treated by monoclonal antibodies, The Journal of Infectious Diseases, doi:10.1093/infdis/jiad523.
Bruhn et al., Somatic hypermutation shapes the viral escape profile of SARS-CoV-2 neutralising antibodies, eBioMedicine, doi:10.1016/j.ebiom.2025.105770.
Ngiam et al., Early administration of neutralising monoclonal antibodies and post-acute sequelae of COVID-19, International Journal of Infectious Diseases, doi:10.1016/j.ijid.2026.108435.
Choudhary et al., Emergence of SARS-CoV-2 Resistance with Monoclonal Antibody Therapy, medRxiv, doi:10.1101/2021.09.03.21263105.
Günther et al., Variant-specific humoral immune response to SARS-CoV-2 escape mutants arising in clinically severe, prolonged infection, medRxiv, doi:10.1101/2024.01.06.24300890.
Casadevall et al., Single monoclonal antibodies should not be used for COVID-19 therapy: a call for antiviral stewardship, Clinical Infectious Diseases, doi:10.1093/cid/ciae408.