Feb 11 |
HH-120 for COVID-19: real-time meta-analysis of 2 studies (Version 2) | |
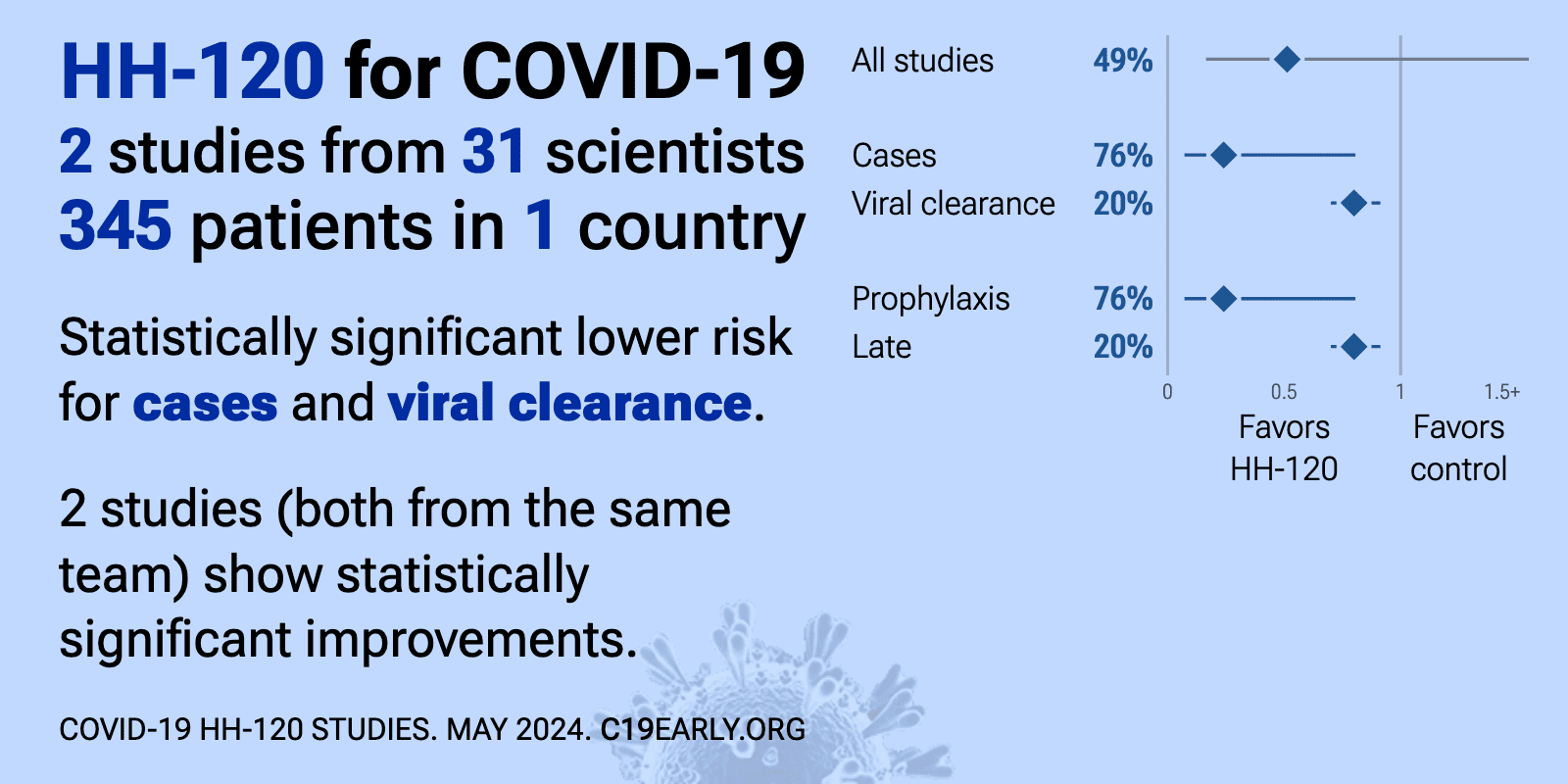
| Significantly lower risk is seen for cases and viral clearance. 2 studies (both from the same team/sponsor) show significant benefit. Meta-analysis using the most serious outcome reported shows 49% [-60‑84%] lower risk, without.. | ||
Dec 6 2023 |
et al., Journal of Medical Virology, doi:10.1002/jmv.29275 | Nasal spray of an IgM‐like ACE2 fusion protein HH‐120 prevents SARS‐CoV‐2 infection: Two investigator‐initiated postexposure prophylaxis trials |
| 76% fewer symptomatic cases (p=0.02) and 58% fewer cases (p=0.03). RCT 269 participants showing significantly reduced risk of infection and symptomatic infection with IgM-like ACE2 fusion protein HH-120 nasal spray used as post-exposure prophylaxis. Participants self-administered HH-120 or placebo 5-10 t.. | ||
May 25 2023 |
et al., Journal of Medical Virology, doi:10.1002/jmv.28805 | Nasal spray of an IgM‐like ACE2 fusion protein HH‐120 accelerates SARS‐CoV‐2 clearance: A single‐center propensity score‐matched cohort study |
| 20% faster viral clearance (p=0.001). PSM analysis of 65 HH-120 patients and 103 controls contemporaneously hospitalized in the same hospital, showing faster viral clearance with HH-120 treatment, with improved results for patients with higher baseline viral load. | ||