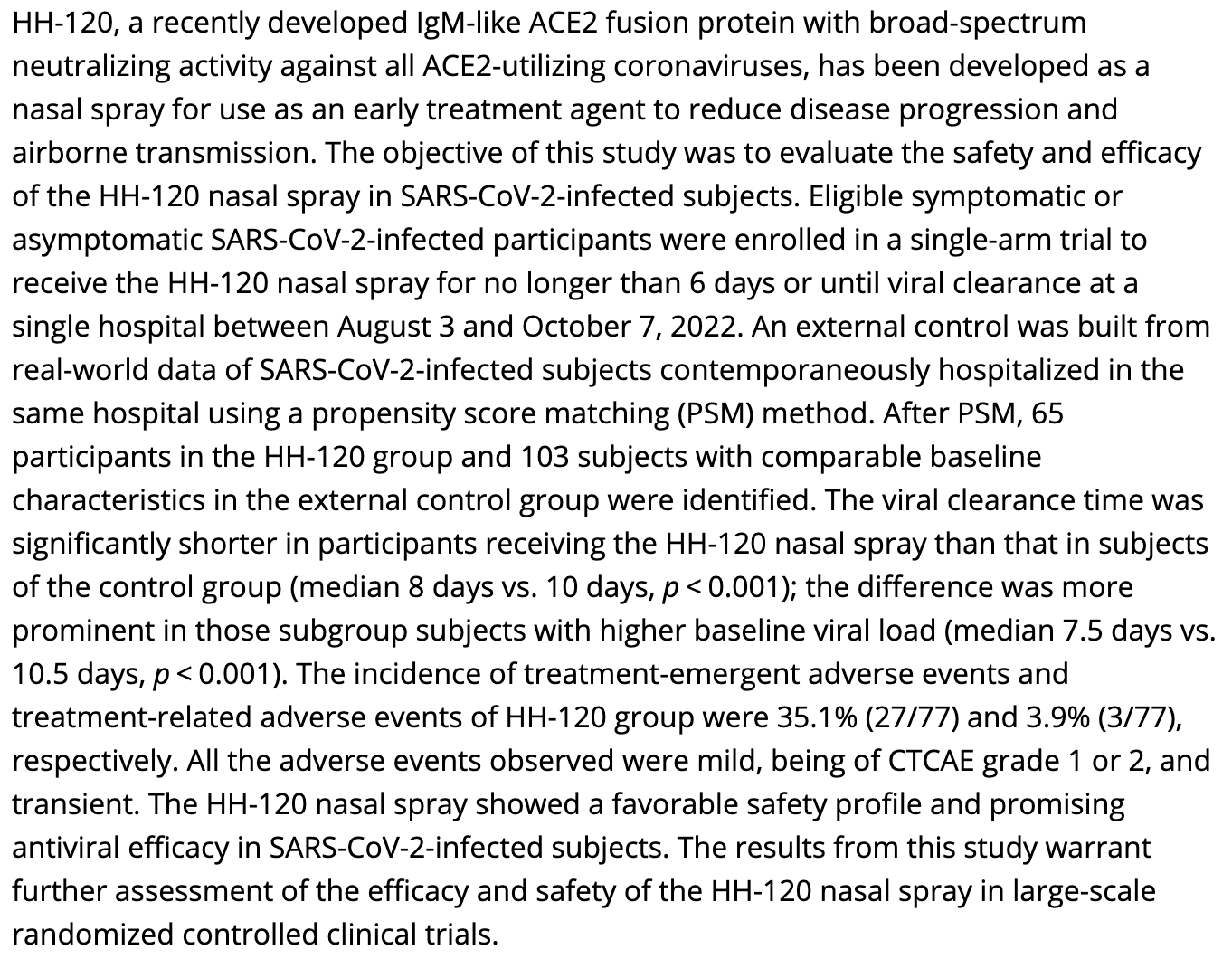
Nasal spray of an IgM‐like ACE2 fusion protein HH‐120 accelerates SARS‐CoV‐2 clearance: A single‐center propensity score‐matched cohort study
et al., Journal of Medical Virology, doi:10.1002/jmv.28805, May 2023
PSM analysis of 65 HH-120 patients and 103 controls contemporaneously hospitalized in the same hospital, showing faster viral clearance with HH-120 treatment, with improved results for patients with higher baseline viral load.
Standard of Care (SOC) for COVID-19 in the study country,
China, is average with moderate efficacy for approved treatments1.
|
time to viral-, 20.0% lower, relative time 0.80, p = 0.001, treatment 65, control 103, propensity score matching.
|
|
time to viral-, 28.6% lower, relative time 0.71, p = 0.001, higher baseline viral load, propensity score matching.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Song et al., 25 May 2023, retrospective, China, peer-reviewed, 13 authors, study period 3 August, 2022 - 7 October, 2022.
DOI record:
{
"DOI": "10.1002/jmv.28805",
"ISSN": [
"0146-6615",
"1096-9071"
],
"URL": "http://dx.doi.org/10.1002/jmv.28805",
"abstract": "<jats:title>Abstract</jats:title><jats:sec><jats:label /><jats:p>HH‐120, a recently developed IgM‐like ACE2 fusion protein with broad‐spectrum neutralizing activity against all ACE2‐utilizing coronaviruses, has been developed as a nasal spray for use as an early treatment agent to reduce disease progression and airborne transmission. The objective of this study was to evaluate the safety and efficacy of the HH‐120 nasal spray in SARS‐CoV‐2‐infected subjects. Eligible symptomatic or asymptomatic SARS‐CoV‐2‐infected participants were enrolled in a single‐arm trial to receive the HH‐120 nasal spray for no longer than 6 days or until viral clearance at a single hospital between August 3 and October 7, 2022. An external control was built from real‐world data of SARS‐CoV‐2‐infected subjects contemporaneously hospitalized in the same hospital using a propensity score matching (PSM) method. After PSM, 65 participants in the HH‐120 group and 103 subjects with comparable baseline characteristics in the external control group were identified. The viral clearance time was significantly shorter in participants receiving the HH‐120 nasal spray than that in subjects of the control group (median 8 days vs. 10 days, <jats:italic>p</jats:italic> < 0.001); the difference was more prominent in those subgroup subjects with higher baseline viral load (median 7.5 days vs. 10.5 days, <jats:italic>p</jats:italic> < 0.001). The incidence of treatment‐emergent adverse events and treatment‐related adverse events of HH‐120 group were 35.1% (27/77) and 3.9% (3/77), respectively. All the adverse events observed were mild, being of CTCAE grade 1 or 2, and transient. The HH‐120 nasal spray showed a favorable safety profile and promising antiviral efficacy in SARS‐CoV‐2‐infected subjects. The results from this study warrant further assessment of the efficacy and safety of the HH‐120 nasal spray in large‐scale randomized controlled clinical trials.</jats:p></jats:sec>",
"alternative-id": [
"10.1002/jmv.28805"
],
"assertion": [
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Received",
"name": "received",
"order": 0,
"value": "2023-02-27"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Accepted",
"name": "accepted",
"order": 1,
"value": "2023-05-08"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Published",
"name": "published",
"order": 2,
"value": "2023-05-25"
}
],
"author": [
{
"affiliation": [
{
"name": "Beijing Ditan Hospital Capital Medical University Beijing China"
}
],
"family": "Song",
"given": "Rui",
"sequence": "first"
},
{
"affiliation": [
{
"name": "Beijing Ditan Hospital Capital Medical University Beijing China"
}
],
"family": "Chen",
"given": "Xiaoyou",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Beijing Ditan Hospital Capital Medical University Beijing China"
}
],
"family": "Li",
"given": "Baoliang",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Huahui Health Ltd. Beijing China"
}
],
"family": "Zhang",
"given": "Hongbin",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Beijing Ditan Hospital Capital Medical University Beijing China"
}
],
"family": "Guo",
"given": "Xiaodi",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Beijing Ditan Hospital Capital Medical University Beijing China"
}
],
"family": "Liu",
"given": "Zhe",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Huahui Health Ltd. Beijing China"
}
],
"family": "Zou",
"given": "Liangfeng",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Huahui Health Ltd. Beijing China"
}
],
"family": "Liang",
"given": "Xiao",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Huahui Health Ltd. Beijing China"
}
],
"family": "Lei",
"given": "Cong",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Huahui Health Ltd. Beijing China"
}
],
"family": "Mao",
"given": "Fengfeng",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "National Institute of Biological Sciences Beijing China"
},
{
"name": "Tsinghua Institute of Multidisciplinary Biomedical Research Tsinghua University Beijing China"
}
],
"family": "Sui",
"given": "Jianhua",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "National Institute of Biological Sciences Beijing China"
},
{
"name": "Tsinghua Institute of Multidisciplinary Biomedical Research Tsinghua University Beijing China"
}
],
"family": "Li",
"given": "Wenhui",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Beijing Ditan Hospital Capital Medical University Beijing China"
},
{
"name": "National Center for Infectious Diseases, Beijing Ditan Hospital Capital Medical University Beijing China"
},
{
"name": "Institute of Infectious Diseases, Beijing Key Laboratory of Emerging Infectious Diseases, Beijing Ditan Hospital Capital Medical University Beijing China"
}
],
"family": "Jin",
"given": "Ronghua",
"sequence": "additional"
}
],
"container-title": "Journal of Medical Virology",
"container-title-short": "Journal of Medical Virology",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"onlinelibrary.wiley.com"
]
},
"created": {
"date-parts": [
[
2023,
5,
25
]
],
"date-time": "2023-05-25T11:54:04Z",
"timestamp": 1685015644000
},
"deposited": {
"date-parts": [
[
2023,
8,
20
]
],
"date-time": "2023-08-20T08:31:37Z",
"timestamp": 1692520297000
},
"indexed": {
"date-parts": [
[
2023,
12,
6
]
],
"date-time": "2023-12-06T18:08:50Z",
"timestamp": 1701886130182
},
"is-referenced-by-count": 2,
"issue": "5",
"issued": {
"date-parts": [
[
2023,
5
]
]
},
"journal-issue": {
"issue": "5",
"published-print": {
"date-parts": [
[
2023,
5
]
]
}
},
"language": "en",
"license": [
{
"URL": "http://onlinelibrary.wiley.com/termsAndConditions#vor",
"content-version": "vor",
"delay-in-days": 24,
"start": {
"date-parts": [
[
2023,
5,
25
]
],
"date-time": "2023-05-25T00:00:00Z",
"timestamp": 1684972800000
}
}
],
"link": [
{
"URL": "https://onlinelibrary.wiley.com/doi/pdf/10.1002/jmv.28805",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "311",
"original-title": [],
"prefix": "10.1002",
"published": {
"date-parts": [
[
2023,
5
]
]
},
"published-online": {
"date-parts": [
[
2023,
5,
25
]
]
},
"published-print": {
"date-parts": [
[
2023,
5
]
]
},
"publisher": "Wiley",
"reference": [
{
"DOI": "10.1056/NEJMoa2034577",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_2_1"
},
{
"DOI": "10.1056/NEJMoa2029849",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_3_1"
},
{
"DOI": "10.1056/NEJMoa2107934",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_4_1"
},
{
"DOI": "10.1056/NEJMoa2035002",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_5_1"
},
{
"DOI": "10.1056/NEJMoa2116846",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_6_1"
},
{
"DOI": "10.1056/NEJMoa2118542",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_7_1"
},
{
"DOI": "10.1056/NEJMoa2116044",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_8_1"
},
{
"key": "e_1_2_9_9_1",
"unstructured": "WHO. 2023. Weekly epidemiological update on COVID‐19 ‐ 22 February. Accessed February 27 2023. https://www.who.int/publications/m/item/weekly-epidemiological-update-on-covid-19---22-february-2023."
},
{
"DOI": "10.1038/s41586-021-04388-0",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_10_1"
},
{
"DOI": "10.1016/S1473-3099(22)00663-6",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_11_1"
},
{
"article-title": "Imprinted SARS‐CoV‐2 humoral immunity induces convergent Omicron RBD evolution",
"author": "Cao Y",
"first-page": "521",
"issue": "7948",
"journal-title": "Nature",
"key": "e_1_2_9_12_1",
"volume": "614",
"year": "2023"
},
{
"DOI": "10.1016/j.cell.2022.12.018",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_13_1"
},
{
"DOI": "10.1101/2022.12.27.521986",
"doi-asserted-by": "crossref",
"key": "e_1_2_9_14_1",
"unstructured": "TamuraT ItoJ UriuK et al.Virological characteristics of the SARS‐CoV‐2 XBB variant derived from recombination of two Omicron subvariants.bioRxiv. Preprint posted online December 27 2022.doi:10.1101/2022.12.27.521986"
},
{
"DOI": "10.1038/s41586-020-2012-7",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_15_1"
},
{
"DOI": "10.1016/j.cell.2020.02.052",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_16_1"
},
{
"DOI": "10.1038/nature02145",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_17_1"
},
{
"DOI": "10.1126/science.1116480",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_18_1"
},
{
"DOI": "10.1038/s41586-022-05513-3",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_19_1"
},
{
"DOI": "10.1126/scitranslmed.abn7737",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_20_1"
},
{
"DOI": "10.15252/emmm.202216109",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_21_1"
},
{
"DOI": "10.1126/science.abe0075",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_22_1"
},
{
"DOI": "10.1016/j.cell.2020.04.004",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_23_1"
},
{
"DOI": "10.1038/s41467-021-24013-y",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_24_1"
},
{
"DOI": "10.1016/j.tips.2022.06.011",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_25_1"
},
{
"DOI": "10.1126/sciadv.abq6527",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_26_1"
},
{
"DOI": "10.21203/rs.3.rs-2044084/v1",
"doi-asserted-by": "crossref",
"key": "e_1_2_9_27_1",
"unstructured": "LiW LiuJ MaoF et al.An IgM‐like inhalable ACE2 fusion protein broadly neutralizes SARS‐CoV‐2.Research Square. Preprint posted online October 31 2022.doi:10.21203/rs.3.rs-2044084/v1"
},
{
"DOI": "10.4049/jimmunol.154.5.2226",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_28_1"
},
{
"DOI": "10.1016/j.cell.2022.11.030",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_29_1"
},
{
"DOI": "10.1007/s11684-022-0981-7",
"doi-asserted-by": "crossref",
"key": "e_1_2_9_30_1",
"unstructured": "LuG LingY JiangM et al.Primary assessment of the diversity of omicron sublineages and the epidemiologic features of autumn/winter 2022 COVID‐19 wave in Chinese mainland.Front Med.2023:1‐10.https://doi.org/10.1007/s11684-022-0981-7"
},
{
"key": "e_1_2_9_31_1",
"unstructured": "US Department of Health and Human Services. Common terminology criteria for adverse events. Version 5.0. November 27 2017."
},
{
"DOI": "10.18637/jss.v042.i08",
"doi-asserted-by": "crossref",
"key": "e_1_2_9_32_1",
"unstructured": "HoDE ImaiK KingG StuartEA.MatchIt: nonparametric preprocessing for parametric causal inference.J Stat Softw.2011;42(8)."
},
{
"DOI": "10.3390/vaccines10091409",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_33_1"
},
{
"DOI": "10.1080/22221751.2022.2078230",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_34_1"
},
{
"DOI": "10.7554/eLife.81849",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_35_1"
},
{
"DOI": "10.1007/s00405-020-06457-y",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_36_1"
},
{
"DOI": "10.1126/scitranslmed.abl7430",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_37_1"
},
{
"DOI": "10.1111/irv.13095",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_38_1"
},
{
"DOI": "10.1016/S2666-5247(23)00011-3",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_39_1"
},
{
"DOI": "10.1016/j.cell.2022.05.005",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_40_1"
},
{
"DOI": "10.1016/S0140-6736(22)00327-0",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_41_1"
},
{
"DOI": "10.1126/science.abf4896",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_42_1"
},
{
"DOI": "10.1056/NEJMc2209651",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_43_1"
},
{
"DOI": "10.1136/gutjnl-2021-326792",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_44_1"
},
{
"DOI": "10.1016/j.aohep.2022.100763",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_45_1"
}
],
"reference-count": 44,
"references-count": 44,
"relation": {},
"resource": {
"primary": {
"URL": "https://onlinelibrary.wiley.com/doi/10.1002/jmv.28805"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Infectious Diseases",
"Virology"
],
"subtitle": [],
"title": "Nasal spray of an IgM‐like ACE2 fusion protein HH‐120 accelerates SARS‐CoV‐2 clearance: A single‐center propensity score‐matched cohort study",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1002/crossmark_policy",
"volume": "95"
}