Camostat was adopted
in 1 country.
Feb 21 |
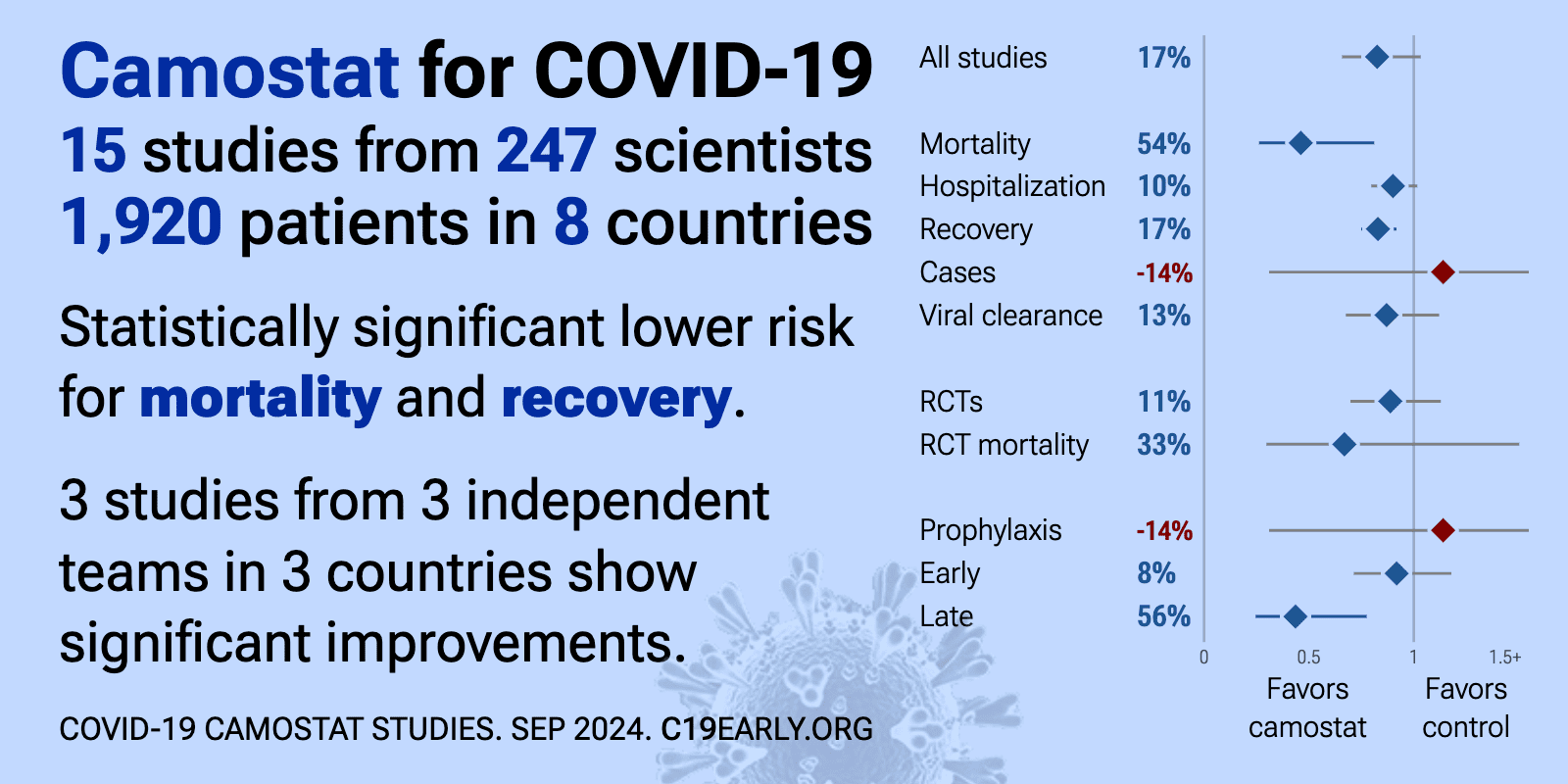
Camostat for COVID-19: real-time meta-analysis of 16 studies (Version 6) | |
| Significantly lower risk is seen for mortality and recovery. 3 studies from 3 independent teams in 3 countries show significant benefit. Meta-analysis using the most serious outcome reported shows 18% [-2‑34%] lower risk, witho.. | ||
Sep 30 2025 |
et al., iScience, doi:10.1016/j.isci.2025.113318 | Evolution of the SARS-CoV-2 spike protein in utilizing host transmembrane serine proteases |
| In vitro study showing variant-dependent differences in SARS-CoV-2 spike protein activation by host transmembrane serine proteases (TTSPs). Authors find that all SARS-CoV-2 variants (Wuhan, Alpha, Beta, Gamma, Delta, and Omicron) require .. | ||
Jun 5 2025 |
et al., Research Square, doi:10.21203/rs.3.rs-6819274/v1 | INP-Guided Network Pharmacology Discloses Multi-Target Therapeutic Strategy Against Cytokine and IgE Storms in the SARS-CoV-2 NB.1.8.1 Variant |
| Computational modeling study presenting a multi-target therapeutic approach against the SARS-CoV-2 NB.1.8.1 variant using Intrinsic Network Pharmacology (INP) and network pharmacology tools. The researchers identified ZINC000014930714, a .. | ||
Feb 12 2025 |
et al., Viruses, doi:10.3390/v17020252 | Inhaled Dry Powder of Antiviral Agents: A Promising Approach to Treating Respiratory Viral Pathogens |
| Review of inhaled dry powder antiviral formulations for treating respiratory viral infections, focusing on COVID-19. Authors explain that traditional antiviral tablets face limitations including systemic side effects and delayed onset of .. | ||
Mar 28 2024 |
et al., NCT04470544 | RECOVER: Phase 2 Randomized, Double-Blind Trial TREating Hospitalized Patients With COVID-19 With Camostat MesilatE, a TMPRSS2 Inhibitor |
| 25% lower mortality (p=1). RCT 100 patients showing no significant difference with camostat. Results are currently unclear - different mortality numbers were provided for all-cause mortality and mortality rate (2/50 vs. 3/46 for the treatment group at 28 days, with.. | ||
Feb 14 2024 |
et al., ACS Omega, doi:10.1021/acsomega.3c06968 | Biophysical Analysis of Potential Inhibitors of SARS-CoV-2 Cell Recognition and Their Effect on Viral Dynamics in Different Cell Types: A Computational Prediction from In Vitro Experimental Data |
| In silico study showing that nafamostat, camostat, chloroquine, hydroxychloroquine, telmisartan, and captopril may be beneficial for COVID-19 by inhibiting SARS-CoV-2 cell entry and replication in multiple cell types expressing ACE2 and T.. | ||
Nov 20 2023 |
et al., BJGP Open, doi:10.3399/bjgpo.2023.0109 | The DAWN antivirals trial: process evaluation of a COVID-19 trial in general practice |
| 33% improved recovery (p=0.7). Small early terminated RCT showing better recovery with camostat treatment, without statistical significance. | ||
Jun 5 2023 |
et al., Clinical Infectious Diseases, doi:10.1093/cid/ciad342 | One Week of Oral Camostat Versus Placebo in Nonhospitalized Adults With Mild-to-Moderate Coronavirus Disease 2019 (COVID-19): A Randomized Controlled Phase 2 Trial |
| 18% higher hospitalization (p=1), 10% lower progression (p=0.11), and 13% improved recovery (p=0.7). RCT 216 patients, 55% >5 days from symptom onset, showing no significant difference with camostat treatment. Longer-term mortality results are from the registry and are not shown in the paper. | ||
Jan 24 2023 |
et al., Antimicrobial Agents and Chemotherapy, doi:10.1128/aac.00452-22 | A Double-Blind, Randomized, Placebo-Controlled, Phase II Clinical Study To Evaluate the Efficacy and Safety of Camostat Mesylate (DWJ1248) in Adult Patients with Mild to Moderate COVID-19 |
| 8% faster recovery (p=0.54). Double-blind RCT with 342 mild to moderate COVID-19 outpatients in South Korea, showing no significant difference in time to clinical improvement with camostat mesylate. In a post-hoc subgroup analysis of high-risk patients, there were.. | ||
Sep 30 2022 |
et al., International Journal of Infectious Diseases, doi:10.1016/j.ijid.2022.06.054 | Efficacy and safety of camostat mesylate in early COVID-19 disease in an ambulatory setting: a randomized placebo-controlled phase II trial |
| 36% higher hospitalization (p=1) and 8% improved recovery (p=0.84). RCT 90 outpatients showing no significant difference in viral load or time to clinical improvement with camostat mesylate. The trial was discontinued early and did not reach the intended sample size. Authors note that combining camostat w.. | ||
Sep 27 2022 |
et al., BMC Medicine, doi:10.1186/s12916-022-02518-7 | A multicenter, double-blind, randomized, parallel-group, placebo-controlled study to evaluate the efficacy and safety of camostat mesilate in patients with COVID-19 (CANDLE study) |
| 67% lower progression (p=1), 50% lower need for oxygen therapy (p=0.37), 1% worse recovery (p=1), and 1% worse viral clearance (p=0.97). RCT 155 hospitalized patients showing no significant differences with camostat. | ||
Jul 22 2022 |
et al., Frontiers in Pharmacology, doi:10.3389/fphar.2022.870493 | Camostat Mesylate Versus Lopinavir/Ritonavir in Hospitalized Patients With COVID-19—Results From a Randomized, Controlled, Open Label, Platform Trial (ACOVACT) |
| 72% lower mortality (p=0.1), 70% lower ventilation (p=0.02), 60% lower combined mortality/intubation (p=0.04), and 18% faster recovery (p=0.005). RCT 201 hospitalized COVID-19 patients showing faster clinical improvement, less progression to mechanical ventilation or death, and shorter hospital stay with camostat mesylate compared to lopinavir/ritonavir. There was also a trend towa.. | ||
Jun 30 2022 |
et al., Jurnal Teknologi Laboratorium, doi:10.29238/teknolabjournal.v11i1.344 | Inhibitory potentials of ivermectin, nafamostat, and camostat on spike protein and some nonstructural proteins of SARS-CoV-2: Virtual screening approach |
| In silico study of ivermectin, camostat, and nafamostat, showing that ivermectin had the best inhibitory action on the SARS-CoV-2 spike protein and Nsp10, while nafamostat had the best results for the other non-structural proteins. Author.. | ||
Jun 3 2022 |
et al., eClinicalMedicine, doi:10.1016/j.eclinm.2022.101484 | Favipiravir, camostat, and ciclesonide combination therapy in patients with moderate COVID-19 pneumonia with/without oxygen therapy: An open-label, single-center phase 3 randomized clinical trial |
| 8% lower progression (p=1) and 40% higher hospital discharge (p=0.04). RCT 117 hospitalized patients with moderate COVID-19 pneumonia in Japan, showing a shorter time to discharge with favipiravir, camostat, and ciclesonide combination therapy compared to favipiravir monotherapy. Subgroup analysis showed gre.. | ||
Mar 3 2022 |
et al., NCT04455815 | A Randomised Phase II Trial in Early COVID-19, Assessing Use of Camostat by Blocking SARS-CoV-2 Spike Protein-initiated Membrane Fusion |
| 14% lower hospitalization (p=1). Early terminated RCT with 34 patients showing no significant differences with camostat treatment. | ||
Feb 7 2022 |
et al., Nature, doi:10.1038/s41586-022-04482-x | Pyrimidine inhibitors synergize with nucleoside analogues to block SARS-CoV-2 |
| In vitro and mouse study showing synergistic antiviral effects when combining pyrimidine biosynthesis inhibitors with antiviral nucleoside analogues against SARS-CoV-2. Authors screened 18 thousand drugs and validated 122 with antiviral a.. | ||
Jan 31 2022 |
et al., medRxiv, doi:10.1101/2022.01.28.22270035 | A Phase 2 Randomized, Double-Blind, Placebo-controlled Trial of Oral Camostat Mesylate for Early Treatment of COVID-19 Outpatients Showed Shorter Illness Course and Attenuation of Loss of Smell and Taste |
| 37% improved recovery (p=0.15). RCT 70 outpatients showing significantly lower symptom scores at day 6, faster recovery, and improved taste/smell, and fatigue with camostat treatment. There was no significant difference for viral load. The recovery result is from.. | ||
Jan 12 2022 |
et al., bioRxiv, doi:10.1101/2022.01.11.475889 | Favipiravir, umifenovir and camostat mesylate: a comparative study against SARS-CoV-2 |
| In vitro and in silico study showing that the combination of favipiravir and umifenovir or camostat mesylate has greater antiviral efficacy than single drug treatment. | ||
Dec 31 2021 |
et al., Chemical Science, doi:10.1039/D1SC01494C | Synergistic inhibition of SARS-CoV-2 cell entry by otamixaban and covalent protease inhibitors: pre-clinical assessment of pharmacological and molecular properties |
| In vitro study showing that otamixaban inhibits SARS-CoV-2 cell entry through TMPRSS2 inhibition. Authors found otamixaban to be a weak TMPRSS2 inhibitor in cell culture (IC50 of 18.7 μM) compared to camostat (IC50 of 151.1 nM) and nafamo.. | ||
Dec 9 2022 |
et al., NCT04713176 | A Double-blind, Randomized, Placebo-controlled, Multi-center, Phase III Study to Evaluate the Efficacy and Safety of DW1248 With Remdesivir in Severe COVID-19 Patients |
| 240 patient camostat late treatment RCT with results not reported over 3 years after completion. | ||
Dec 2 2021 |
et al., NCT04608266 | A Multicenter Randomized Trial to Evaluate the Efficacy and Safety of Camostat Mesylate for the Treatment of SARS-CoV-2 Infection - COVID-19 in Ambulatory Adult Patients (CAMOVID) |
| 70 patient camostat early treatment RCT with results not reported over 4 years after completion. | ||
Oct 29 2021 |
et al., NCT04681430 | Reconvalescent Plasma / Camostat Mesylate Early in Sars-CoV-2 Q-PCR (COVID-19) Positive High-risk Individuals |
| 22 patient camostat early treatment RCT with results not reported over 4 years after completion. | ||
Jun 10 2021 |
et al., NCT04530617 | Randomized, Double-blind, Placebo-controlled, Multicenter, Multi-arm, Phase II Trial of Novel Agents for the Treatment of Mild to Moderate COVID-19 Positive Outpatients |
| 246 patient camostat early treatment RCT with results not reported over 4 years after completion. | ||
May 31 2021 |
et al., eClinicalMedicine, doi:10.1016/j.eclinm.2021.100849 | Efficacy of the TMPRSS2 inhibitor camostat mesilate in patients hospitalized with Covid-19-a double-blind randomized controlled trial |
| 18% lower mortality (p=0.75), 31% lower ventilation (p=0.65), 20% lower ICU admission (p=0.61), and 15% improved recovery (p=0.28). RCT 205 hospitalized patients showing no significant benefit with camostat. There was a trend towards lower risk of ICU admission or death in the camostat group (10% vs. 18% for placebo), but the study was not powered for this endpoint. V.. | ||
May 15 2021 |
et al., NCT04524663 | A Phase 2 Randomized, Double Blinded, Placebo Controlled Study of Oral Camostat Mesilate Compared to Standard of Care in Subjects With Mild-Moderate COVID-19 |
| 35% improved recovery (p=0.24), 86% improvement (p=0.11), and 41% improved viral clearance (p=0.24). RCT 49 outpatients in the USA, showing no significant differences with camostat treatment. | ||
Apr 30 2021 |
et al., Frontiers in Molecular Biosciences, doi:10.3389/fmolb.2021.666626 | Computational Identification of a Putative Allosteric Binding Pocket in TMPRSS2 |
| In silico study of TMPRSS2 inhibition by camostat, nafamostat, and bromhexine, suggesting allosteric binding for bromhexine, compared to camostat and nafamostat which bind to the active site of TMPRSS2 forming covalent adducts. | ||
Apr 12 2021 |
et al., Intensive Care Medicine, doi:10.1007/s00134-021-06395-1 | Camostat mesylate therapy in critically ill patients with COVID-19 pneumonia |
| 69% lower mortality (p=0.001), 10% lower ventilation (p=1), and 17% longer hospitalization (p=0.35). Retrospective 371 critically ill COVID-19 patients showing lower mortality with camostat mesylate treatment. | ||
Mar 31 2021 |
, NCT04583592 | A Multicenter, Randomized, Double-Blind, Placebo-Controlled Trial of the Efficacy of Camostat Mesilate for Treatment of COVID-19 in Outpatients |
| 152% higher mortality (p=1), 13% lower progression (p=0.79), and 16% improved viral clearance (p=0.36). RCT 295 outpatients in the USA, showing no significant differences with camostat. | ||
Dec 19 2020 |
et al., International Journal of Infectious Diseases, doi:10.1016/j.ijid.2020.12.041 | Association of prescribed medications with the risk of COVID-19 infection and severity among adults in South Korea |
| 14% more cases (p=0.84). Retrospective database analysis showing no significant differences with camostat use. | ||
Dec 11 2020 |
et al., NCT04355052 | Open Label Study to Compare Efficacy, Safety and Tolerability of Hydroxychloroquine Combined With Azithromycin Compared to Hydroxychloroquine Combined With Camostat Mesylate and to "no Treatment" in SARS CoV 2 Virus (COSTA) |
| Estimated 250 patient camostat late treatment RCT with results not reported over 5 years after estimated completion. | ||
Nov 16 2020 |
et al., Critical Care Explorations, doi:10.1097/CCE.0000000000000284 | Camostat Mesylate May Reduce Severity of Coronavirus Disease 2019 Sepsis: A First Observation |
| 58% lower mortality (p=0.55), 61% shorter ICU admission, and 56% improved recovery. Retrospective 11 critically ill COVID-19 ICU patients with organ failure treated with camostat mesylate (6 patients) or HCQ (5 patients). Over an 8 day period, the severity of COVID-19 decreased in the camostat group as measured by a decl.. | ||
Mar 5 2020 |
et al., Cell, doi:10.1016/j.cell.2020.02.052 | SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor |
| In vitro study showing that SARS-CoV-2 uses ACE2 for entry and TMPRSS2 for S protein priming, and that TMPRSS2 inhibitor camostat blocked entry and may be an effective treaetment. | ||