Feb 27 |
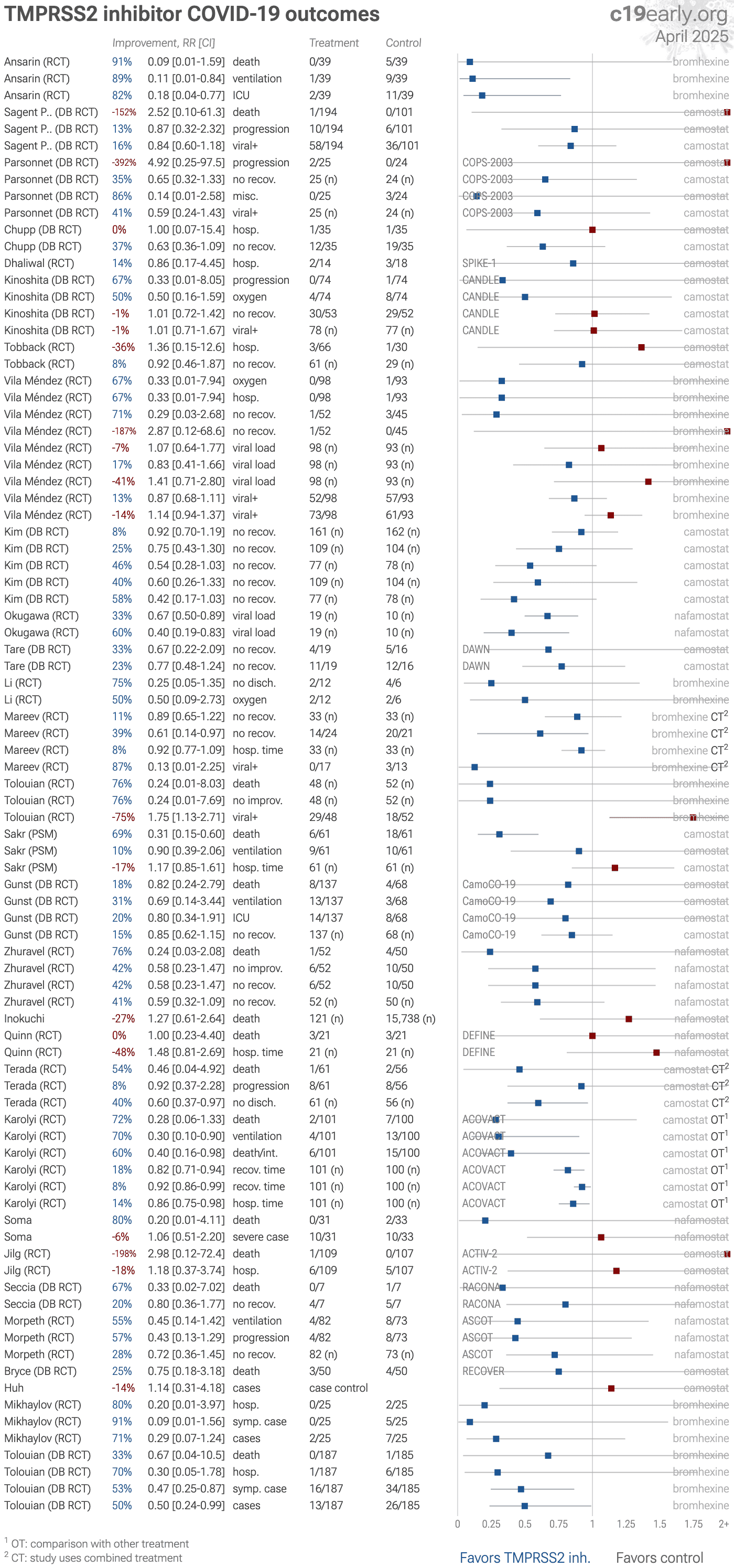
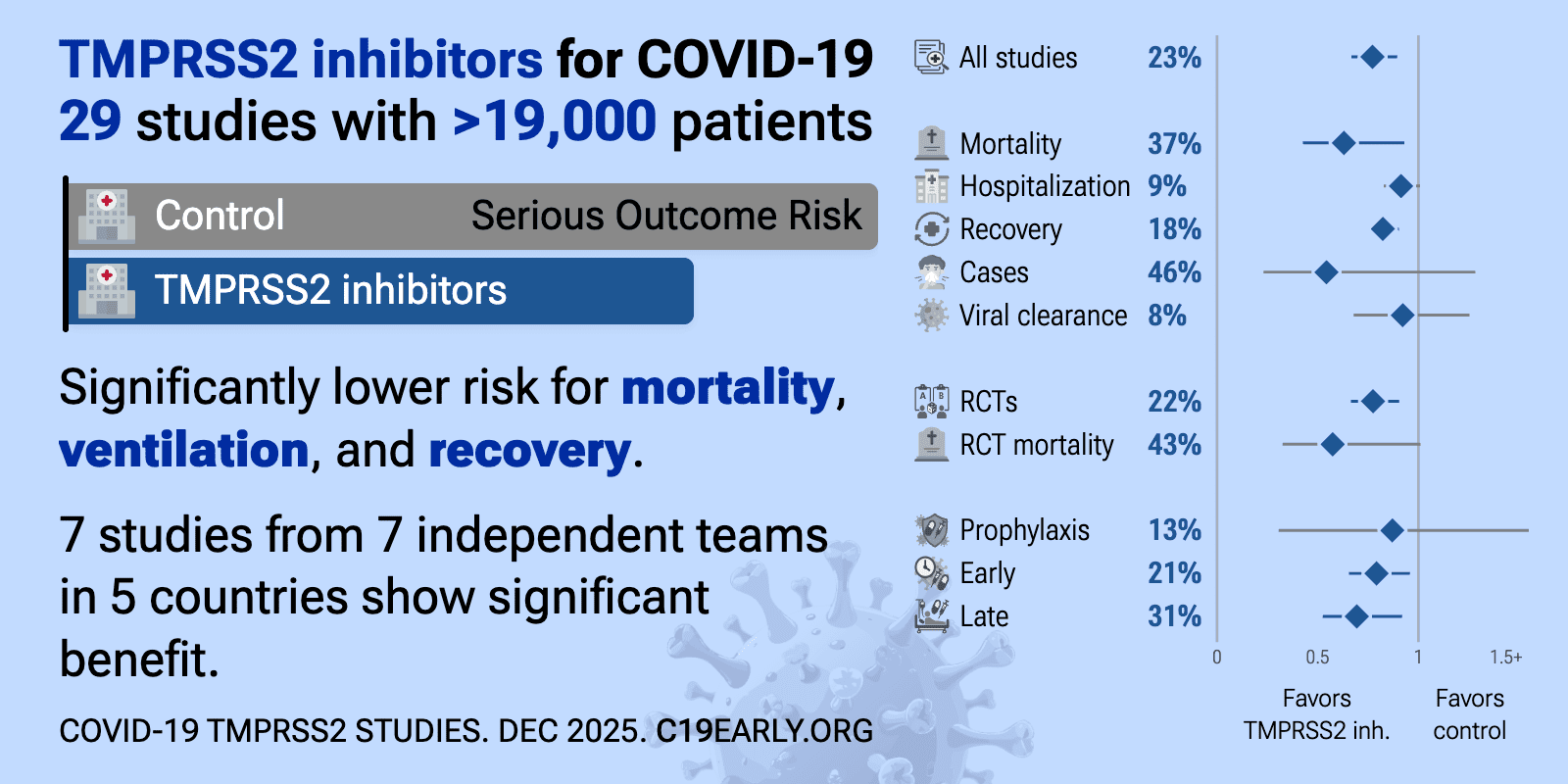
TMPRSS2 inhibitors reduce COVID-19 risk: real-time meta-analysis of 29 studies | |
| Significantly lower risk is seen for mortality, ventilation, and recovery. 7 studies from 7 independent teams in 5 countries show significant benefit. Meta-analysis using the most serious outcome reported shows 23% [10‑33%] low.. | ||
Feb 12 2025 |
et al., Viruses, doi:10.3390/v17020252 | Inhaled Dry Powder of Antiviral Agents: A Promising Approach to Treating Respiratory Viral Pathogens |
| Review of inhaled dry powder antiviral formulations for treating respiratory viral infections, focusing on COVID-19. Authors explain that traditional antiviral tablets face limitations including systemic side effects and delayed onset of .. | ||
Jan 31 2025 |
et al., Scientifica, doi:10.1155/sci5/3687892 | Exploring TMPRSS2 Drug Target to Combat Influenza and Coronavirus Infection |
| Review of TMPRSS2 as a drug target to combat influenza and coronavirus infections. Authors highlight that TMPRSS2, a transmembrane serine protease coexpressed with ACE2 receptors in the human respiratory tract, plays a critical role in vi.. | ||
Nov 21 2024 |
et al., Discover Molecules, doi:10.1007/s44345-024-00005-5 | Exploring potential therapeutic candidates against COVID-19: a molecular docking study |
| In silico study showing potential inhibition of SARS-CoV-2 proteins by various compounds including dactinomycin, itraconazole, ivermectin, vitamin D, quercetin, curcumin, montelukast, bromhexine, hesperidin, EGCG and raloxifene. Authors p.. | ||
Oct 25 2024 |
et al., Preprints.org, doi:10.20944/preprints202410.1998.v1 | COVID-19 Prophylactic Effect of Bromhexine Hydrochloride |
| Retrospective 125 outpatients showing reduced COVID-19 infection rates with prophylactic bromhexine hydrochloride (BRH) use during 2021-2022 COVID waves in Bulgaria. Prior to BRH prophylaxis, 62% of participants reported confirmed COVID-1.. | ||
Mar 28 2024 |
et al., NCT04470544 | RECOVER: Phase 2 Randomized, Double-Blind Trial TREating Hospitalized Patients With COVID-19 With Camostat MesilatE, a TMPRSS2 Inhibitor |
| 25% lower mortality (p=1). RCT 100 patients showing no significant difference with camostat. Results are currently unclear - different mortality numbers were provided for all-cause mortality and mortality rate (2/50 vs. 3/46 for the treatment group at 28 days, with.. | ||
Mar 28 2024 |
et al., International Journal of Nanomedicine, doi:10.2147/IJN.S448005 | Lipid Nanoparticle-Based Inhibitors for SARS-CoV-2 Host Cell Infection |
| In vitro study showing that lactoferrin, camostat mesylate, and carrageenan inhibit SARS-CoV-2 pseudovirus infection in airway epithelial Calu-3 cells. All show dose-dependent inhibition. The study focuses on novel LNP formulations and t.. | ||
Mar 14 2024 |
et al., eClinicalMedicine, 10.1016/j.eclinm.2024.102517 | Early treatment with fluvoxamine, bromhexine, cyproheptadine, and niclosamide to prevent clinical deterioration in patients with symptomatic COVID-19: a randomized clinical trial |
| 98% lower ventilation (p<0.0001), 100% lower need for oxygen therapy (p<0.0001), 98% lower hospitalization (p<0.0001), and 55% lower long COVID (p<0.0001). RCT 995 outpatients showing significantly lower progression with early treatment within 48 hours using fluvoxamine, fluvoxamine+bromhexine, fluvoxamine+cyproheptadine, and niclosamide+bromhexine. 70% of patients received treatment within .. | ||
Feb 14 2024 |
et al., ACS Omega, doi:10.1021/acsomega.3c06968 | Biophysical Analysis of Potential Inhibitors of SARS-CoV-2 Cell Recognition and Their Effect on Viral Dynamics in Different Cell Types: A Computational Prediction from In Vitro Experimental Data |
| In silico study showing that nafamostat, camostat, chloroquine, hydroxychloroquine, telmisartan, and captopril may be beneficial for COVID-19 by inhibiting SARS-CoV-2 cell entry and replication in multiple cell types expressing ACE2 and T.. | ||
Nov 20 2023 |
et al., BJGP Open, doi:10.3399/bjgpo.2023.0109 | The DAWN antivirals trial: process evaluation of a COVID-19 trial in general practice |
| 33% improved recovery (p=0.7). Small early terminated RCT showing better recovery with camostat treatment, without statistical significance. | ||
Oct 24 2023 |
et al., NEJM Evidence, doi:10.1056/EVIDoa2300132 | A Randomized Trial of Nafamostat for Covid-19 |
| 55% lower ventilation (p=0.23), 57% lower progression (p=0.14), and 28% improved recovery (p=0.36). RCT 160 hospitalized non-critically ill COVID-19 patients showing a 93% posterior probability that nafamostat reduced the odds of death or receipt of ventilatory or vasopressor support by day 28 compared to usual care. Nafamostat, a TMPRS.. | ||
Oct 20 2023 |
, V., Pharmacia, doi:10.3897/pharmacia.70.e112550 | Comparison of treatment of COVID-19 with inhaled bromhexine, higher doses of colchicine and hymecromone with WHO-recommended paxlovid, molnupiravir, remdesivir, anti-IL-6 receptor antibodies and baricitinib |
| Review of treatment of COVID-19 with inhaled bromhexine, higher doses of colchicine and hymecromone compared to WHO-recommended paxlovid, molnupiravir, remdesivir, anti-IL-6 receptor antibodies, and baricitinib. The author argues that the.. | ||
Oct 19 2023 |
et al., Journal of Clinical Medicine, doi:10.3390/jcm12206618 | RAndomized Clinical Trial Of NAfamostat Mesylate, A Potent Transmembrane Protease Serine 2 (TMPRSS2) Inhibitor, in Patients with COVID-19 Pneumonia |
| 67% lower mortality (p=1) and 20% improved recovery (p=1). RCT 15 hospitalized COVID-19 patients showing a positive safety profile with nafamostat mesylate treatment. While the study was underpowered to detect differences in efficacy, Bayesian analysis suggested a signal for potential benefit (69.. | ||
Sep 30 2023 |
et al., International Journal of Antimicrobial Agents, doi:10.1016/j.ijantimicag.2023.106922 | Antiviral effect and safety of nafamostat mesilate in patients with mild early-onset COVID-19: An exploratory multicentre randomized controlled clinical trial |
| 33% improved viral clearance (p=0.007). RCT 30 early-onset COVID-19 patients showing significantly improved viral load reduction with nafamostat. | ||
Jun 5 2023 |
et al., Clinical Infectious Diseases, doi:10.1093/cid/ciad342 | One Week of Oral Camostat Versus Placebo in Nonhospitalized Adults With Mild-to-Moderate Coronavirus Disease 2019 (COVID-19): A Randomized Controlled Phase 2 Trial |
| 18% higher hospitalization (p=1), 10% lower progression (p=0.11), and 13% improved recovery (p=0.7). RCT 216 patients, 55% >5 days from symptom onset, showing no significant difference with camostat treatment. Longer-term mortality results are from the registry and are not shown in the paper. | ||
Feb 8 2023 |
et al., NCT04390594 | Multicentre, Open Label, Randomised, Adaptative Clinical Trial of Efficacy and Safety of Treatment Regimens in Adult COVID-19 Patients in Senegal |
| 59 patient TMPRSS2 inhibitor late treatment RCT with results not reported over 3 years after completion. | ||
Jan 24 2023 |
et al., Antimicrobial Agents and Chemotherapy, doi:10.1128/aac.00452-22 | A Double-Blind, Randomized, Placebo-Controlled, Phase II Clinical Study To Evaluate the Efficacy and Safety of Camostat Mesylate (DWJ1248) in Adult Patients with Mild to Moderate COVID-19 |
| 8% faster recovery (p=0.54). Double-blind RCT with 342 mild to moderate COVID-19 outpatients in South Korea, showing no significant difference in time to clinical improvement with camostat mesylate. In a post-hoc subgroup analysis of high-risk patients, there were.. | ||
Jan 3 2023 |
et al., Research Square, doi:10.21203/rs.3.rs-2309373/v2 | Evaluation of the recovery rate and prevention of hospitalization among covid-19 outpatients: a randomized clinical trial comparing N-acetylcysteine with Bromhexine |
| 93% lower mortality (p=0.01), 88% lower hospitalization (p<0.0001), and 28% faster recovery (p<0.0001). RCT 225 outpatients in Iran showing lower mortality and hospitalization, and faster recovery with N-acetylcysteine and bromhexine. Baseline information per group is not provided, Figure 1 has the control group hospitalization status switc.. | ||
Dec 26 2022 |
et al., bioRxiv, doi:10.1101/2022.12.23.521817 | In Vitro Inhibition of SARS-CoV-2 Infection by Bromhexine hydrochloride |
| In vitro study showing that bromhexine inhibits SARS-CoV-2 infection and replication in vitro by blocking the host cell protease TMPRSS2. | ||
Dec 24 2022 |
et al., Journal of Clinical Medicine, doi:10.3390/jcm12010142 | Efficacy of Bromhexine versus Standard of Care in Reducing Viral Load in Patients with Mild-to-Moderate COVID-19 Disease Attended in Primary Care: A Randomized Open-Label Trial |
| 67% lower hospitalization (p=0.49) and 7% worse viral clearance (p=0.82). RCT 191 low risk (no mortality) outpatients in Spain, showing no significant differences with bromhexine. Authors note that "statistical differences between the study groups were observed in the percentage of patients treated with.. | ||
Sep 30 2022 |
et al., International Journal of Infectious Diseases, doi:10.1016/j.ijid.2022.06.054 | Efficacy and safety of camostat mesylate in early COVID-19 disease in an ambulatory setting: a randomized placebo-controlled phase II trial |
| 36% higher hospitalization (p=1) and 8% improved recovery (p=0.84). RCT 90 outpatients showing no significant difference in viral load or time to clinical improvement with camostat mesylate. The trial was discontinued early and did not reach the intended sample size. Authors note that combining camostat w.. | ||
Sep 30 2022 |
et al., Japanese Journal of Infectious Diseases, doi:10.7883/yoken.JJID.2021.699 | Nafamostat Mesylate Monotherapy in Patients with Moderate COVID-19: a Single-Center, Retrospective Study |
| 80% lower mortality (p=0.49) and 6% higher severe cases (p=1). Retrospective 64 hospitalized patients with moderate COVID-19 showing no significant difference in clinical outcomes with nafamostat mesylate. | ||
Sep 27 2022 |
et al., BMC Medicine, doi:10.1186/s12916-022-02518-7 | A multicenter, double-blind, randomized, parallel-group, placebo-controlled study to evaluate the efficacy and safety of camostat mesilate in patients with COVID-19 (CANDLE study) |
| 67% lower progression (p=1), 50% lower need for oxygen therapy (p=0.37), 1% worse recovery (p=1), and 1% worse viral clearance (p=0.97). RCT 155 hospitalized patients showing no significant differences with camostat. | ||
Aug 1 2022 |
et al., NCT04871646 | A Double-blind, Multi-center, Multi-regional, Randomized Controlled, Phase 3 Clinical Trial to Evaluate the Efficacy and Safety of CKD-314 in Hospitalized Adult Patients Diagnosed With COVID-19 |
| Estimated 586 patient TMPRSS2 inhibitor late treatment RCT with results not reported over 3 years after estimated completion. | ||
Jul 22 2022 |
et al., Frontiers in Pharmacology, doi:10.3389/fphar.2022.870493 | Camostat Mesylate Versus Lopinavir/Ritonavir in Hospitalized Patients With COVID-19—Results From a Randomized, Controlled, Open Label, Platform Trial (ACOVACT) |
| 72% lower mortality (p=0.1), 70% lower ventilation (p=0.02), 60% lower combined mortality/intubation (p=0.04), and 18% faster recovery (p=0.005). RCT 201 hospitalized COVID-19 patients showing faster clinical improvement, less progression to mechanical ventilation or death, and shorter hospital stay with camostat mesylate compared to lopinavir/ritonavir. There was also a trend towa.. | ||
Jun 30 2022 |
et al., Jurnal Teknologi Laboratorium, doi:10.29238/teknolabjournal.v11i1.344 | Inhibitory potentials of ivermectin, nafamostat, and camostat on spike protein and some nonstructural proteins of SARS-CoV-2: Virtual screening approach |
| In silico study of ivermectin, camostat, and nafamostat, showing that ivermectin had the best inhibitory action on the SARS-CoV-2 spike protein and Nsp10, while nafamostat had the best results for the other non-structural proteins. Author.. | ||
Jun 3 2022 |
et al., eClinicalMedicine, doi:10.1016/j.eclinm.2022.101484 | Favipiravir, camostat, and ciclesonide combination therapy in patients with moderate COVID-19 pneumonia with/without oxygen therapy: An open-label, single-center phase 3 randomized clinical trial |
| 8% lower progression (p=1) and 40% higher hospital discharge (p=0.04). RCT 117 hospitalized patients with moderate COVID-19 pneumonia in Japan, showing a shorter time to discharge with favipiravir, camostat, and ciclesonide combination therapy compared to favipiravir monotherapy. Subgroup analysis showed gre.. | ||
Mar 3 2022 |
et al., NCT04455815 | A Randomised Phase II Trial in Early COVID-19, Assessing Use of Camostat by Blocking SARS-CoV-2 Spike Protein-initiated Membrane Fusion |
| 14% lower hospitalization (p=1). Early terminated RCT with 34 patients showing no significant differences with camostat treatment. | ||
Feb 28 2022 |
et al., eBioMedicine, doi:10.1016/j.ebiom.2022.103856 | Randomised controlled trial of intravenous nafamostat mesylate in COVID pneumonitis: Phase 1b/2a experimental study to investigate safety, Pharmacokinetics and Pharmacodynamics |
| 48% longer hospitalization (p=0.21). RCT 42 hospitalized patients with COVID-19 pneumonitis showing no benefit with intravenous nafamostat mesylate. | ||
Feb 7 2022 |
et al., Nature, doi:10.1038/s41586-022-04482-x | Pyrimidine inhibitors synergize with nucleoside analogues to block SARS-CoV-2 |
| In vitro and mouse study showing synergistic antiviral effects when combining pyrimidine biosynthesis inhibitors with antiviral nucleoside analogues against SARS-CoV-2. Authors screened 18 thousand drugs and validated 122 with antiviral a.. | ||
Jan 31 2022 |
et al., medRxiv, doi:10.1101/2022.01.28.22270035 | A Phase 2 Randomized, Double-Blind, Placebo-controlled Trial of Oral Camostat Mesylate for Early Treatment of COVID-19 Outpatients Showed Shorter Illness Course and Attenuation of Loss of Smell and Taste |
| 37% improved recovery (p=0.15). RCT 70 outpatients showing significantly lower symptom scores at day 6, faster recovery, and improved taste/smell, and fatigue with camostat treatment. There was no significant difference for viral load. The recovery result is from.. | ||
Jan 12 2022 |
et al., bioRxiv, doi:10.1101/2022.01.11.475889 | Favipiravir, umifenovir and camostat mesylate: a comparative study against SARS-CoV-2 |
| In vitro and in silico study showing that the combination of favipiravir and umifenovir or camostat mesylate has greater antiviral efficacy than single drug treatment. | ||
Dec 31 2021 |
et al., Chemical Science, doi:10.1039/D1SC01494C | Synergistic inhibition of SARS-CoV-2 cell entry by otamixaban and covalent protease inhibitors: pre-clinical assessment of pharmacological and molecular properties |
| In vitro study showing that otamixaban inhibits SARS-CoV-2 cell entry through TMPRSS2 inhibition. Authors found otamixaban to be a weak TMPRSS2 inhibitor in cell culture (IC50 of 18.7 μM) compared to camostat (IC50 of 151.1 nM) and nafamo.. | ||
Dec 26 2021 |
et al., Journal of Clinical Medicine, doi:10.3390/jcm11010116 | Association between Nafamostat Mesylate and In-Hospital Mortality in Patients with Coronavirus Disease 2019: A Multicenter Observational Study |
| 27% higher mortality (p=0.52). Retrospective multicenter observational study of 15,859 hospitalized COVID-19 patients in Japan showing no significant difference in in-hospital mortality with nafamostat mesylate. Very few patients received treatment and they had more se.. | ||
Dec 20 2021 |
et al., SSRN, doi:10.2139/ssrn.3989 | Bromhexine, for Post Exposure COVID-19 Prophylaxis: A Randomized, Double-Blind, Placebo Control Trial |
| 70% lower hospitalization (p=0.15), 53% fewer symptomatic cases (p=0.007), and 50% fewer cases (p=0.03). PEP RCT with 372 close contacts of COVID+ patients, 187 treated with bromhexine, showing significantly lower cases with treatment. IRCT20120703010178N22. | ||
Dec 2 2021 |
et al., NCT04608266 | A Multicenter Randomized Trial to Evaluate the Efficacy and Safety of Camostat Mesylate for the Treatment of SARS-CoV-2 Infection - COVID-19 in Ambulatory Adult Patients (CAMOVID) |
| 70 patient TMPRSS2 inhibitor early treatment RCT with results not reported over 4 years after completion. | ||
Nov 30 2021 |
et al., eClinicalMedicine, doi:10.1016/j.eclinm.2021.101169 | Nafamostat in hospitalized patients with moderate to severe COVID-19 pneumonia: a randomised Phase II clinical trial |
| 76% lower mortality (p=0.2), 42% greater improvement (p=0.28), and 42% improved recovery (p=0.28). RCT 104 hospitalized patients with moderate to severe COVID-19 pneumonia showing no significant difference in the primary endpoint of time to clinical improvement with nafamostat. However, in patients with baseline National Early Warning .. | ||
Jun 30 2021 |
et al., BMJ Open, doi:10.1136/bmjopen-2020-045190 | New prophylaxis regimen for SARS-CoV-2 infection in health professionals with low doses of hydroxychloroquine and bromhexine: a randomised, double-blind placebo clinical trial (ELEVATE Trial) |
| Estimated 214 participant TMPRSS2 inhibitor + HCQ prophylaxis RCT with results not reported over 4 years after estimated completion. | ||
May 31 2021 |
et al., eClinicalMedicine, doi:10.1016/j.eclinm.2021.100849 | Efficacy of the TMPRSS2 inhibitor camostat mesilate in patients hospitalized with Covid-19-a double-blind randomized controlled trial |
| 18% lower mortality (p=0.75), 31% lower ventilation (p=0.65), 20% lower ICU admission (p=0.61), and 15% improved recovery (p=0.28). RCT 205 hospitalized patients showing no significant benefit with camostat. There was a trend towards lower risk of ICU admission or death in the camostat group (10% vs. 18% for placebo), but the study was not powered for this endpoint. V.. | ||
May 15 2021 |
et al., NCT04524663 | A Phase 2 Randomized, Double Blinded, Placebo Controlled Study of Oral Camostat Mesilate Compared to Standard of Care in Subjects With Mild-Moderate COVID-19 |
| 35% improved recovery (p=0.24), 86% improvement (p=0.11), and 41% improved viral clearance (p=0.24). RCT 49 outpatients in the USA, showing no significant differences with camostat treatment. | ||
Apr 30 2021 |
et al., Frontiers in Molecular Biosciences, doi:10.3389/fmolb.2021.666626 | Computational Identification of a Putative Allosteric Binding Pocket in TMPRSS2 |
| In silico study of TMPRSS2 inhibition by camostat, nafamostat, and bromhexine, suggesting allosteric binding for bromhexine, compared to camostat and nafamostat which bind to the active site of TMPRSS2 forming covalent adducts. | ||
Apr 30 2021 |
et al., NCT04418128 | Treatment Effect of Nafamostat Mesylate in Patients With COVID-19 Pneumonia: Open Labelled Randomized Controlled Clinical Trial |
| Estimated 84 patient TMPRSS2 inhibitor late treatment RCT with results not reported over 4 years after estimated completion. | ||
Apr 12 2021 |
et al., Intensive Care Medicine, doi:10.1007/s00134-021-06395-1 | Camostat mesylate therapy in critically ill patients with COVID-19 pneumonia |
| 69% lower mortality (p=0.001), 10% lower ventilation (p=1), and 17% longer hospitalization (p=0.35). Retrospective 371 critically ill COVID-19 patients showing lower mortality with camostat mesylate treatment. | ||
Apr 5 2021 |
et al., NCT04628143 | Open-label, Multi-center, Randomized Controlled, Phase 2 Clinical Trial to Evaluate the Efficacy and Safety of CKD-314 in Hospitalized Adult Patients Diagnosed With COVID-19 |
| 13 patient TMPRSS2 inhibitor late treatment RCT with results not reported over 4 years after completion. | ||
Mar 31 2021 |
, NCT04583592 | A Multicenter, Randomized, Double-Blind, Placebo-Controlled Trial of the Efficacy of Camostat Mesilate for Treatment of COVID-19 in Outpatients |
| 152% higher mortality (p=1), 13% lower progression (p=0.79), and 16% improved viral clearance (p=0.36). RCT 295 outpatients in the USA, showing no significant differences with camostat. | ||
Mar 15 2021 |
et al., J. Investig. Med., doi:10.1136/jim-2020-001747 | Effect of bromhexine in hospitalized patients with COVID-19 |
| 76% lower mortality (p=0.43), 76% greater improvement (p=0.43), and 75% worse viral clearance (p=0.02). Small RCT with 100 patients, 48 with bromhexine added to SOC, showing slower viral- conversion but lower mortality and greater clinical improvement with bromhexine (not statistically significant with few deaths and very high recovery). Th.. | ||
Mar 8 2021 |
et al., Interdisciplinary Perspectives on Infectious Diseases, doi:10.1155/2022/4693121 (date from preprint) | Bromhexine Hydrochloride Prophylaxis of COVID-19 for Medical Personnel: A Randomized Open-Label Study |
| 91% fewer symptomatic cases (p=0.05) and 71% fewer cases (p=0.14). Small prophylaxis RCT with 25 treatment and 25 control health care workers, showing lower PCR+, symptomatic cases, and hospitalization with treatment, although not statistically significant with the small sample size. | ||
Jan 31 2021 |
et al., Journal of Biological Chemistry, doi:10.1016/j.jbc.2021.100701 | Inhibition of acid sphingomyelinase by ambroxol prevents SARS-CoV-2 entry into epithelial cells |
| In vitro study showing that ambroxol (a metabolite of bromhexine) inhibits SARS-CoV-2 infection. | ||
Dec 31 2020 |
et al., Current Medical and Drug Research | The potential role of Bromhexine in the management of COVID-19: Decipher and a real game-changer |
| Review article noting that bromhexine is a TMPRSS2 inhibitor with greater effect in lung tissue and attenuates the entry and proliferation of SARS-CoV-2. | ||
Dec 19 2020 |
et al., International Journal of Infectious Diseases, doi:10.1016/j.ijid.2020.12.041 | Association of prescribed medications with the risk of COVID-19 infection and severity among adults in South Korea |
| 14% more cases (p=0.84). Retrospective database analysis showing no significant differences with camostat use. | ||
Dec 3 2020 |
et al., Кардиология, doi:10.18087/cardio.2020.11.n1440 | Results of Open-Label non-Randomized Comparative Clinical Trial: “BromhexIne and Spironolactone for CoronаvirUs Infection requiring hospiTalization (BISCUIT) |
| 11% improved recovery (p=0.47), 8% shorter hospitalization (p=0.35), and 87% improved viral clearance (p=0.08). Prospective 103 PCR+ patients in Russia, 33 treated with bromexhine+spironolactone, showing lower PCR+ at day 10 or hospitalization >10 days with treatment. Bromhexine 8mg 4 times daily, spironolactone 25-50 mg/day for 10 days. | ||
Sep 3 2020 |
et al., Clinical and Translational Science, doi:10.1111/cts.12881 | Bromhexine Hydrochloride Tablets for the Treatment of Moderate COVID-19: An Open-Label Randomized Controlled Pilot Study |
| 75% higher hospital discharge (p=0.11) and 3% slower recovery. Tiny RCT with 12 bromhexine and 6 control patients showing non-statistically significant improvements in chest CT, need for oxygen therapy, and discharge rate within 20 days. Authors recommend a larger scale trial. | ||
Jul 31 2020 |
et al., NCT04355026 | Use of Bromhexine and Hydroxychloroquine for Treatment of COVID-19 Pneumonia |
| Estimated 90 patient TMPRSS2 inhibitor + HCQ late treatment RCT with results not reported over 5 years after estimated completion. | ||
Jul 19 2020 |
et al., Bioimpacts, doi:10.34172/bi.2020.27 | Effect of bromhexine on clinical outcomes and mortality in COVID-19 patients: A randomized clinical trial |
| 91% lower mortality (p=0.05), 89% lower ventilation (p=0.01), and 82% lower ICU admission (p=0.01). RCT with 39 bromhexine and 39 control patients showing lower mortality, intubation, and ICU admission with treatment. The treatment group received bromhexine hydrochloride 8 mg three times a day for two weeks. All patients received SOC in.. | ||
May 26 2020 |
et al., Internal and Emergency Medicine, doi:10.1007/s11739-020-02383-3 | Potential new treatment strategies for COVID-19: is there a role for bromhexine as add-on therapy? |
| Proposal to use bromhexine to inhibit TMPRSS2-specific viral entry for prophylaxis and treatment of COVID-19. | ||
Apr 30 2020 |
et al., Pharmacol. Res., doi:10.1016/j.phrs.2020.104853 | Possible use of the mucolytic drug, bromhexine hydrochloride, as a prophylactic agent against SARS-CoV-2 infection based on its action on the Transmembrane Serine Protease 2 |
| Note on the potential use of bromhexine hydrochloride for prophylaxis of SARS-CoV-2, based on the role of TMPRSS2 in SARS-CoV-2 infection, and the TMPRSS2 inhibition of bromhexine hydrochloride. | ||
Apr 22 2020 |
et al., Pharmacol Res., doi:10.1016/j.phrs.2020.104837 | Repurposing the mucolytic cough suppressant and TMPRSS2 protease inhibitor bromhexine for the prevention and management of SARS-CoV-2 infection |
| Proposal to use bromhexine for prophylaxis and treatment of COVID-19 based on TMPRSS2 inhibition, widespread clinical use, and supporting pharmacokinetic and safety data. | ||
Mar 5 2020 |
et al., Cell, doi:10.1016/j.cell.2020.02.052 | SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor |
| In vitro study showing that SARS-CoV-2 uses ACE2 for entry and TMPRSS2 for S protein priming, and that TMPRSS2 inhibitor camostat blocked entry and may be an effective treaetment. | ||