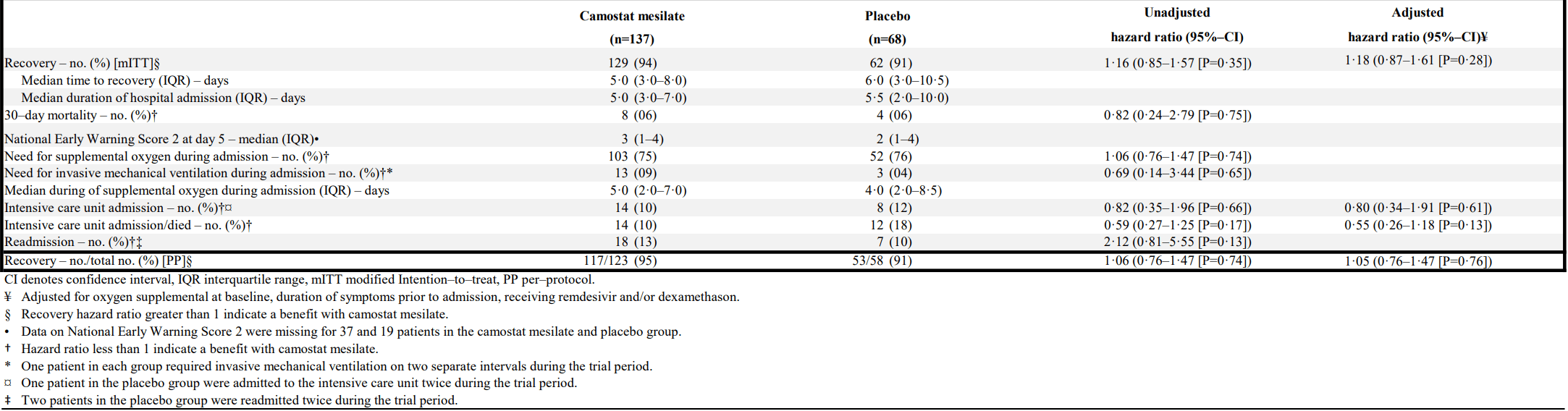
Efficacy of the TMPRSS2 inhibitor camostat mesilate in patients hospitalized with Covid-19-a double-blind randomized controlled trial
et al., eClinicalMedicine, doi:10.1016/j.eclinm.2021.100849, CamoCO-19, NCT04321096, May 2021
22nd treatment shown to reduce risk in
April 2021, now with p = 0.00063 from 29 studies.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
RCT 205 hospitalized patients showing no significant benefit with camostat. There was a trend towards lower risk of ICU admission or death in the camostat group (10% vs. 18% for placebo), but the study was not powered for this endpoint. Viral load and inflammatory markers were not significantly different between groups. The study was underpowered due to faster than expected clinical improvement.
Study covers TMPRSS2 inhibitors and camostat.
|
risk of death, 18.0% lower, HR 0.82, p = 0.75, treatment 8 of 137 (5.8%), control 4 of 68 (5.9%), Cox proportional hazards.
|
|
risk of mechanical ventilation, 31.0% lower, HR 0.69, p = 0.65, treatment 13 of 137 (9.5%), control 3 of 68 (4.4%), Cox proportional hazards.
|
|
risk of ICU admission, 20.0% lower, HR 0.80, p = 0.61, treatment 14 of 137 (10.2%), control 8 of 68 (11.8%), NNT 65, adjusted per study, multivariable, Cox proportional hazards.
|
|
risk of no recovery, 15.3% lower, HR 0.85, p = 0.28, treatment 137, control 68, adjusted per study, inverted to make HR<1 favor treatment, multivariable, Cox proportional hazards.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Gunst et al., 31 May 2021, Double Blind Randomized Controlled Trial, placebo-controlled, Denmark, peer-reviewed, median age 61.0, 39 authors, study period 4 April, 2020 - 31 December, 2020, trial NCT04321096 (history) (CamoCO-19).
Contact: mads@dandrite.au.dk, olesoega@rm.dk.
Efficacy of the TMPRSS2 inhibitor camostat mesilate in patients hospitalized with Covid-19-a double-blind randomized controlled trial.
EClinicalMedicine, doi:10.1016/j.eclinm.2021.100849
Background: The trans-membrane protease serine 2 (TMPRSS2) is essential for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) cell entry and infection. Efficacy and safety of TMPRSS2 inhibitors in patients with coronavirus disease 2019 (Covid-19) have not been evaluated in randomized trials. Methods: We conducted an investigator-initiated, double-blind, randomized, placebo-controlled multicenter trial in patients hospitalized with confirmed SARS-CoV-2 infection from April 4, to December 31, 2020. Within 48 h of admission, participants were randomly assigned in a 2:1 ratio to receive the TMPRSS2 inhibitor camostat mesilate 200 mg three times daily for 5 days or placebo. The primary outcome was time to discharge or clinical improvement measured as 2 points improvement on a 7-point ordinal scale. Other outcomes included 30-day mortality, safety and change in oropharyngeal viral load.
Supplementary materials Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.eclinm.2021.100849.
References
Beigel, Tomashek, Dodd, Remdesivir for the treatment of Covid-19-final report, N Engl J Med
Breining, Frolund, Hojen, Camostat mesylate against SARS-CoV-2 and Covid-19-rationale, dosing and safety, Basic Clin Pharmacol Toxicol
Burke, Midgley, Dratch, Active monitoring of persons exposed to patients with confirmed Covid-19-United States, January-February 2020, MMWR Morb Mortal Wkly Rep
Cao, Wang, Wen, A Trial of lopinavir-ritonavir in adults hospitalized with severe Covid-19, N Engl J Med
Consortium, Pan, Peto, Repurposed antiviral drugs for Covid-19-interim WHO solidarity trial results, N Engl J Med
Doi, Ikeda, Hayase, Moriya, Morimura et al., Nafamostat mesylate treatment in combination with favipiravir for patients critically ill with COVID-19: a case series, Crit Care
Group, Dexamethasone in hospitalized patients with Covid-19-Preliminary report, N Engl J Med
Group, Horby, Lim, Dexamethasone in hospitalized patients with Covid-19-preliminary report, N Engl J Med
Harris, Taylor, Thielke, Payne, Gonzalez et al., Research electronic data capture (REDCap)Àa metadata-driven methodology and workflow process for providing translational research informatics support, J Biomed Inform
Hoffmann, Hofmann-Winkler, Smith, Camostat mesylate inhibits SARS-CoV-2 activation by TMPRSS2-related proteases and its metabolite GBPA exerts antiviral activity, bioRxiv
Hoffmann, Kleine-Weber, Schroeder, SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and Is blocked by a clinically proven protease inhibitor, Cell
Hofmann-Winkler, Moerer, Alt-Epping, Camostat mesylate may reduce severity of coronavirus disease 2019 Sepsis: a first observation, Crit Care Explor
Jang, Rhee, Three cases of treatment with nafamostat in elderly patients with Covid-19 pneumonia who need oxygen therapy, Int J Infect Dis
Joshi, Joshi, Degani, Tackling SARS-CoV-2: proposed targets and repurposed drugs, Future Med Chem
Kim, Read, Fauci, Therapy for early Covid-19: a critical need, JAMA
Libster, Marc, Wappner, Early high-titer plasma therapy to prevent severe Covid-19 in older adults, N Engl J Med
Mahase, Covid-19: WHO declares pandemic because of ''alarming levels'' of spread, severity, and inaction, BMJ
Netea, Rovina, Complex immune dysregulation in Covid-19 patients with severe respiratory failure, Cell Host Microbe
Nihal, Ahmad, Dose translation from animal to human studies revisited, FASEB J
Organization, Who, Coronavirus Disease (COVID-19) Dashboard
Qin, Zhou, Hu, Dysregulation of immune response in patients with Covid-19 in Wuhan, China, Clin Infect Dis
Romagnoli, Peris, Gaudio, Geppetti, SARS-CoV-2 and Covid-19: from the bench to the bedside, Physiol Rev
Stone, Frigault, Nj, Efficacy of tocilizumab in patients hospitalized with Covid-19, N Engl J Med
Talukdar, Saikia, Singal, Tandon, Chronic pancreatitis: evolving paradigms, Pancreatology
Wang, Hu, Hu, Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China, JAMA
Weinreich, Sivapalasingam, Norton, REGN-COV 2 , a neutralizing antibody cocktail, in outpatients with Covid-19, N Engl J Med
Who, Coronavirus disease (COVID-19) R&D
Wu, Mcgoogan, Characteristics of and important lessons from the coronavirus disease 2019 (Covid-19) outbreak in China: summary of a report of 72314 cases from the Chinese center for disease control and prevention, JAMA
Zhou, Vedantham, Lu, Protease inhibitors targeting coronavirus and filovirus entry, Antivir Res
Zhu, Zhang, Wang, A novel coronavirus from patients with pneumonia in China, N Engl J Med
DOI record:
{
"DOI": "10.1016/j.eclinm.2021.100849",
"ISSN": [
"2589-5370"
],
"URL": "http://dx.doi.org/10.1016/j.eclinm.2021.100849",
"alternative-id": [
"S2589537021001292"
],
"article-number": "100849",
"assertion": [
{
"label": "This article is maintained by",
"name": "publisher",
"value": "Elsevier"
},
{
"label": "Article Title",
"name": "articletitle",
"value": "Efficacy of the TMPRSS2 inhibitor camostat mesilate in patients hospitalized with Covid-19-a double-blind randomized controlled trial."
},
{
"label": "Journal Title",
"name": "journaltitle",
"value": "EClinicalMedicine"
},
{
"label": "CrossRef DOI link to publisher maintained version",
"name": "articlelink",
"value": "https://doi.org/10.1016/j.eclinm.2021.100849"
},
{
"label": "Content Type",
"name": "content_type",
"value": "article"
},
{
"label": "Copyright",
"name": "copyright",
"value": "© 2021 The Authors. Published by Elsevier Ltd."
}
],
"author": [
{
"ORCID": "http://orcid.org/0000-0002-3787-0259",
"affiliation": [],
"authenticated-orcid": false,
"family": "Gunst",
"given": "Jesper D.",
"sequence": "first"
},
{
"affiliation": [],
"family": "Staerke",
"given": "Nina B.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Pahus",
"given": "Marie H.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kristensen",
"given": "Lena H.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Bodilsen",
"given": "Jacob",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lohse",
"given": "Nicolai",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Dalgaard",
"given": "Lars S.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Brønnum",
"given": "Dorthe",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Fröbert",
"given": "Ole",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hønge",
"given": "Bo",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Johansen",
"given": "Isik S.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Monrad",
"given": "Ida",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Erikstrup",
"given": "Christian",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Rosendal",
"given": "Regitze",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Vilstrup",
"given": "Emil",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mariager",
"given": "Theis",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Bove",
"given": "Dorthe G.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-5038-4052",
"affiliation": [],
"authenticated-orcid": false,
"family": "Offersen",
"given": "Rasmus",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Shakar",
"given": "Shakil",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Cajander",
"given": "Sara",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Jørgensen",
"given": "Nis P.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Sritharan",
"given": "Sajitha S.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Breining",
"given": "Peter",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-2072-9005",
"affiliation": [],
"authenticated-orcid": false,
"family": "Jespersen",
"given": "Søren",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mortensen",
"given": "Klaus L.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Jensen",
"given": "Mads L.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kolte",
"given": "Lilian",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-7765-6428",
"affiliation": [],
"authenticated-orcid": false,
"family": "Frattari",
"given": "Giacomo S.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Larsen",
"given": "Carsten S.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Storgaard",
"given": "Merete",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Nielsen",
"given": "Lars P.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Tolstrup",
"given": "Martin",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Sædder",
"given": "Eva A.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Østergaard",
"given": "Lars J.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-5948-9929",
"affiliation": [],
"authenticated-orcid": false,
"family": "Ngo",
"given": "Hien T.T.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Jensen",
"given": "Morten H.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Højen",
"given": "Jesper F.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kjolby",
"given": "Mads",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-9107-2023",
"affiliation": [],
"authenticated-orcid": false,
"family": "Søgaard",
"given": "Ole S.",
"sequence": "additional"
}
],
"container-title": "EClinicalMedicine",
"container-title-short": "EClinicalMedicine",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"thelancet.com",
"elsevier.com",
"sciencedirect.com"
]
},
"created": {
"date-parts": [
[
2021,
4,
22
]
],
"date-time": "2021-04-22T09:44:18Z",
"timestamp": 1619084658000
},
"deposited": {
"date-parts": [
[
2022,
7,
24
]
],
"date-time": "2022-07-24T07:30:56Z",
"timestamp": 1658647856000
},
"funder": [
{
"DOI": "10.13039/501100003554",
"doi-asserted-by": "publisher",
"name": "Lundbeck Foundation"
}
],
"indexed": {
"date-parts": [
[
2023,
10,
5
]
],
"date-time": "2023-10-05T17:45:19Z",
"timestamp": 1696527919048
},
"is-referenced-by-count": 128,
"issued": {
"date-parts": [
[
2021,
5
]
]
},
"language": "en",
"license": [
{
"URL": "https://www.elsevier.com/tdm/userlicense/1.0/",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2021,
5,
1
]
],
"date-time": "2021-05-01T00:00:00Z",
"timestamp": 1619827200000
}
},
{
"URL": "http://creativecommons.org/licenses/by-nc-nd/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2021,
3,
31
]
],
"date-time": "2021-03-31T00:00:00Z",
"timestamp": 1617148800000
}
}
],
"link": [
{
"URL": "https://api.elsevier.com/content/article/PII:S2589537021001292?httpAccept=text/xml",
"content-type": "text/xml",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://api.elsevier.com/content/article/PII:S2589537021001292?httpAccept=text/plain",
"content-type": "text/plain",
"content-version": "vor",
"intended-application": "text-mining"
}
],
"member": "78",
"original-title": [],
"page": "100849",
"prefix": "10.1016",
"published": {
"date-parts": [
[
2021,
5
]
]
},
"published-print": {
"date-parts": [
[
2021,
5
]
]
},
"publisher": "Elsevier BV",
"reference": [
{
"DOI": "10.1056/NEJMoa2001017",
"article-title": "A novel coronavirus from patients with pneumonia in China, 2019",
"author": "Zhu",
"doi-asserted-by": "crossref",
"first-page": "727",
"issue": "8",
"journal-title": "N Engl J Med",
"key": "10.1016/j.eclinm.2021.100849_bib0001",
"volume": "382",
"year": "2020"
},
{
"DOI": "10.15585/mmwr.mm6909e1",
"article-title": "Active monitoring of persons exposed to patients with confirmed Covid-19-United States, January-February 2020",
"author": "Burke",
"doi-asserted-by": "crossref",
"first-page": "245",
"issue": "9",
"journal-title": "MMWR Morb Mortal Wkly Rep",
"key": "10.1016/j.eclinm.2021.100849_bib0002",
"volume": "69",
"year": "2020"
},
{
"DOI": "10.1136/bmj.m1036",
"article-title": "Covid-19: WHO declares pandemic because of \"alarming levels\" of spread, severity, and inaction",
"author": "Mahase",
"doi-asserted-by": "crossref",
"first-page": "m1036",
"journal-title": "BMJ",
"key": "10.1016/j.eclinm.2021.100849_bib0003",
"volume": "368",
"year": "2020"
},
{
"key": "10.1016/j.eclinm.2021.100849_bib0004",
"unstructured": "Organization W.H. WHO Coronavirus Disease (COVID-19) Dashboard. 2021. https://covid19.who.int/."
},
{
"DOI": "10.1152/physrev.00020.2020",
"article-title": "SARS-CoV-2 and Covid-19: from the bench to the bedside",
"author": "Romagnoli",
"doi-asserted-by": "crossref",
"first-page": "1455",
"issue": "4",
"journal-title": "Physiol Rev",
"key": "10.1016/j.eclinm.2021.100849_bib0005",
"volume": "100",
"year": "2020"
},
{
"article-title": "Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China",
"author": "Wang",
"journal-title": "JAMA",
"key": "10.1016/j.eclinm.2021.100849_bib0006",
"year": "2020"
},
{
"article-title": "Dysregulation of immune response in patients with Covid-19 in Wuhan, China",
"author": "Qin",
"journal-title": "Clin Infect Dis",
"key": "10.1016/j.eclinm.2021.100849_bib0007",
"year": "2020"
},
{
"DOI": "10.1016/j.chom.2020.04.009",
"article-title": "Complex immune dysregulation in Covid-19 patients with severe respiratory failure",
"author": "Giamarellos-Bourboulis",
"doi-asserted-by": "crossref",
"first-page": "992",
"issue": "6",
"journal-title": "Cell Host Microbe",
"key": "10.1016/j.eclinm.2021.100849_bib0008",
"volume": "27",
"year": "2020"
},
{
"article-title": "Repurposed antiviral drugs for Covid-19-interim WHO solidarity trial results",
"author": "Consortium",
"journal-title": "N Engl J Med",
"key": "10.1016/j.eclinm.2021.100849_bib0009",
"year": "2020"
},
{
"DOI": "10.1056/NEJMoa2028836",
"article-title": "Efficacy of tocilizumab in patients hospitalized with Covid-19",
"author": "Stone",
"doi-asserted-by": "crossref",
"first-page": "2333",
"issue": "24",
"journal-title": "N Engl J Med",
"key": "10.1016/j.eclinm.2021.100849_bib0010",
"volume": "383",
"year": "2020"
},
{
"DOI": "10.1056/NEJMoa2001282",
"article-title": "A Trial of lopinavir-ritonavir in adults hospitalized with severe Covid-19",
"author": "Cao",
"doi-asserted-by": "crossref",
"journal-title": "N Engl J Med",
"key": "10.1016/j.eclinm.2021.100849_bib0011",
"year": "2020"
},
{
"DOI": "10.1056/NEJMoa2007764",
"article-title": "Remdesivir for the treatment of Covid-19-final report",
"author": "Beigel",
"doi-asserted-by": "crossref",
"first-page": "1813",
"issue": "19",
"journal-title": "N Engl J Med",
"key": "10.1016/j.eclinm.2021.100849_bib0012",
"volume": "383",
"year": "2020"
},
{
"article-title": "REGN-COV2, a neutralizing antibody cocktail, in outpatients with Covid-19",
"author": "Weinreich",
"journal-title": "N Engl J Med",
"key": "10.1016/j.eclinm.2021.100849_bib0013",
"year": "2020"
},
{
"article-title": "Dexamethasone in hospitalized patients with Covid-19-Preliminary report",
"author": "Group",
"journal-title": "N Engl J Med",
"key": "10.1016/j.eclinm.2021.100849_bib0014",
"year": "2020"
},
{
"DOI": "10.4155/fmc-2020-0147",
"article-title": "Tackling SARS-CoV-2: proposed targets and repurposed drugs",
"author": "Joshi",
"doi-asserted-by": "crossref",
"first-page": "1579",
"issue": "17",
"journal-title": "Future Med Chem",
"key": "10.1016/j.eclinm.2021.100849_bib0015",
"volume": "12",
"year": "2020"
},
{
"DOI": "10.1001/jama.2020.22813",
"article-title": "Therapy for early Covid-19: a critical need",
"author": "Kim",
"doi-asserted-by": "crossref",
"first-page": "2149",
"issue": "21",
"journal-title": "JAMA",
"key": "10.1016/j.eclinm.2021.100849_bib0016",
"volume": "324",
"year": "2020"
},
{
"DOI": "10.1016/j.cell.2020.02.052",
"article-title": "SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and Is blocked by a clinically proven protease inhibitor",
"author": "Hoffmann",
"doi-asserted-by": "crossref",
"journal-title": "Cell",
"key": "10.1016/j.eclinm.2021.100849_bib0017",
"year": "2020"
},
{
"DOI": "10.1111/bcpt.13533",
"article-title": "Camostat mesylate against SARS-CoV-2 and Covid-19-rationale, dosing and safety",
"author": "Breining",
"doi-asserted-by": "crossref",
"first-page": "204",
"journal-title": "Basic Clin Pharmacol Toxicol",
"key": "10.1016/j.eclinm.2021.100849_bib0018",
"volume": "128(2)",
"year": "2021"
},
{
"DOI": "10.1016/j.antiviral.2015.01.011",
"article-title": "Protease inhibitors targeting coronavirus and filovirus entry",
"author": "Zhou",
"doi-asserted-by": "crossref",
"first-page": "76",
"journal-title": "Antivir Res",
"key": "10.1016/j.eclinm.2021.100849_bib0019",
"volume": "116",
"year": "2015"
},
{
"DOI": "10.1101/2020.08.05.237651",
"doi-asserted-by": "crossref",
"key": "10.1016/j.eclinm.2021.100849_bib0020",
"unstructured": "Hoffmann M., Hofmann-Winkler H., Smith J.C., et al. Camostat mesylate inhibits SARS-CoV-2 activation by TMPRSS2-related proteases and its metabolite GBPA exerts antiviral activity. bioRxiv 2020."
},
{
"DOI": "10.1159/000094561",
"article-title": "Chronic pancreatitis: evolving paradigms",
"author": "Talukdar",
"doi-asserted-by": "crossref",
"first-page": "440",
"issue": "5",
"journal-title": "Pancreatology",
"key": "10.1016/j.eclinm.2021.100849_bib0021",
"volume": "6",
"year": "2006"
},
{
"DOI": "10.1096/fj.07-9574LSF",
"article-title": "Dose translation from animal to human studies revisited",
"author": "Reagan-Shaw",
"doi-asserted-by": "crossref",
"first-page": "659",
"issue": "3",
"journal-title": "FASEB J",
"key": "10.1016/j.eclinm.2021.100849_bib0022",
"volume": "22",
"year": "2008"
},
{
"DOI": "10.1097/CCE.0000000000000284",
"article-title": "Camostat mesylate may reduce severity of coronavirus disease 2019 Sepsis: a first observation",
"author": "Hofmann-Winkler",
"doi-asserted-by": "crossref",
"first-page": "e0284",
"issue": "11",
"journal-title": "Crit Care Explor",
"key": "10.1016/j.eclinm.2021.100849_bib0023",
"volume": "2",
"year": "2020"
},
{
"DOI": "10.1016/j.jbi.2008.08.010",
"article-title": "Research electronic data capture (REDCap)–a metadata-driven methodology and workflow process for providing translational research informatics support",
"author": "Harris",
"doi-asserted-by": "crossref",
"first-page": "377",
"issue": "2",
"journal-title": "J Biomed Inform",
"key": "10.1016/j.eclinm.2021.100849_bib0024",
"volume": "42",
"year": "2009"
},
{
"key": "10.1016/j.eclinm.2021.100849_bib0025",
"unstructured": "WHO. Coronavirus disease (COVID-19) R&D. http://origin.who.int/blueprint/priority-diseases/key-action/novel-coronavirus/en/."
},
{
"article-title": "Dexamethasone in hospitalized patients with Covid-19-preliminary report",
"author": "Group",
"journal-title": "N Engl J Med",
"key": "10.1016/j.eclinm.2021.100849_bib0026",
"year": "2020"
},
{
"DOI": "10.1001/jama.2020.2648",
"article-title": "Characteristics of and important lessons from the coronavirus disease 2019 (Covid-19) outbreak in China: summary of a report of 72314 cases from the Chinese center for disease control and prevention",
"author": "Wu",
"doi-asserted-by": "crossref",
"first-page": "1239",
"issue": "13",
"journal-title": "JAMA",
"key": "10.1016/j.eclinm.2021.100849_bib0027",
"volume": "323",
"year": "2020"
},
{
"DOI": "10.1186/s13054-020-03078-z",
"article-title": "Nafamostat mesylate treatment in combination with favipiravir for patients critically ill with COVID-19: a case series",
"author": "Doi",
"doi-asserted-by": "crossref",
"first-page": "392",
"issue": "1",
"journal-title": "Crit Care",
"key": "10.1016/j.eclinm.2021.100849_bib0028",
"volume": "24",
"year": "2020"
},
{
"DOI": "10.1016/j.ijid.2020.05.072",
"article-title": "Three cases of treatment with nafamostat in elderly patients with Covid-19 pneumonia who need oxygen therapy",
"author": "Jang",
"doi-asserted-by": "crossref",
"first-page": "500",
"journal-title": "Int J Infect Dis",
"key": "10.1016/j.eclinm.2021.100849_bib0029",
"volume": "96",
"year": "2020"
},
{
"DOI": "10.1056/NEJMoa2033700",
"article-title": "Early high-titer plasma therapy to prevent severe Covid-19 in older adults",
"author": "Libster",
"doi-asserted-by": "crossref",
"first-page": "610",
"issue": "7",
"journal-title": "N Engl J Med",
"key": "10.1016/j.eclinm.2021.100849_bib0030",
"volume": "384",
"year": "2021"
}
],
"reference-count": 30,
"references-count": 30,
"relation": {},
"resource": {
"primary": {
"URL": "https://linkinghub.elsevier.com/retrieve/pii/S2589537021001292"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"General Medicine"
],
"subtitle": [],
"title": "Efficacy of the TMPRSS2 inhibitor camostat mesilate in patients hospitalized with Covid-19-a double-blind randomized controlled trial.",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1016/elsevier_cm_policy",
"volume": "35"
}
