Aug 11 2024 |
, D., Current Topics in Microbiology and Immunology, doi:10.1007/82_2024_268 | Monoclonal Antibody Therapies Against SARS-CoV-2: Promises and Realities |
| Review of monoclonal antibodies for SARS-CoV-2. Author notes that the omicron variant has reset achievements to date. | ||
Aug 8 2024 |
et al., Clinical Infectious Diseases, doi:10.1093/cid/ciae408 | Single monoclonal antibodies should not be used for COVID-19 therapy: a call for antiviral stewardship |
| Review arguing against use of single monoclonal antibodies for COVID-19 treatment, particularly in immunosuppressed patients, due to the risk of rapidly selecting for resistant viral variants. Authors suggest that while monoclonal antibod.. | ||
May 6 2024 |
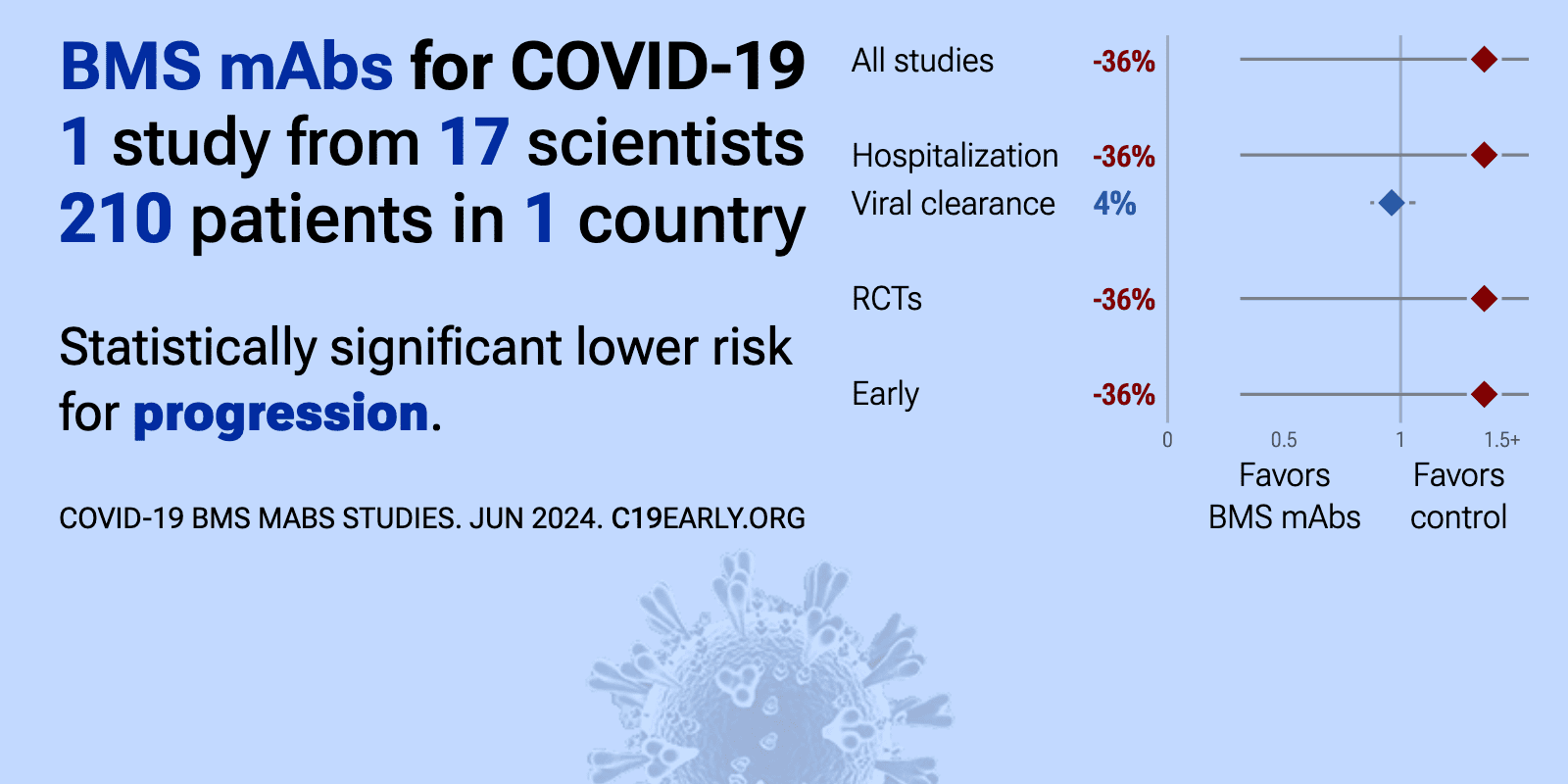
et al., Pathogens & Immunity, doi:10.20411/pai.v9i1.660 | Safety, Efficacy, and Pharmacokinetics of Combination SARS-CoV-2 Neutralizing Monoclonal Antibodies BMS-986414 (C135-LS) and BMS-986413 (C144-LS) Administered Subcutaneously in Non-Hospitalized Persons with COVID-19 in a Phase 2 Trial |
| 17% lower progression (p=0.03), 20% faster recovery (p=0.19), and 4% improved viral clearance (p=0.45). RCT 211 outpatients with COVID-19 showing no significant difference in time to symptom improvement with subcutaneous BMS-986414 (C135-LS, ogalvibart) and BMS-986413 (C144-LS, crexavibart) monoclonal antibodies compared to placebo. There w.. | ||