Safety, Efficacy, and Pharmacokinetics of Combination SARS-CoV-2 Neutralizing Monoclonal Antibodies BMS-986414 (C135-LS) and BMS-986413 (C144-LS) Administered Subcutaneously in Non-Hospitalized Persons with COVID-19 in a Phase 2 Trial
et al., Pathogens & Immunity, doi:10.20411/pai.v9i1.660 , May 2024
RCT 211 outpatients with COVID-19 showing no significant difference in time to symptom improvement with subcutaneous BMS-986414 (C135-LS, ogalvibart) and BMS-986413 (C144-LS, crexavibart) monoclonal antibodies compared to placebo. There was a trend favoring the treatment arm and participants on the monoclonal antibodies were less likely to have worsening symptoms. No significant differences were found for the proportion of patients with undetectable viral load or hospitalization. The study included a low-risk population, which may have limited the treatment effect. Higher early plasma monoclonal antibody concentrations were associated with more favorable outcomes, suggesting the subcutaneous route may not have achieved adequate concentrations quickly enough at the site of infection.
Viral load measured by PCR may not accurately reflect infectious virus measured by viral culture. Porter et al. show that viral load early in infection was correlated with infectious virus, but viral load late in infection could be high even with low or undetectable infectious virus. Assessing viral load later in infection may underestimate reductions in infectious virus with treatment.
Standard of Care (SOC) for COVID-19 in the study country,
the USA, is very poor with very low average efficacy for approved treatments2.
Only expensive, high-profit treatments were approved for early treatment. Low-cost treatments were excluded, reducing the probability of early treatment due to access and cost barriers, and eliminating complementary and synergistic benefits seen with many low-cost treatments.
|
risk of hospitalization, 35.9% higher, RR 1.36, p = 0.72, treatment 4 of 104 (3.8%), control 3 of 106 (2.8%).
|
|
worsening of ≥1 symptom, 17.0% lower, RR 0.83, p = 0.03, treatment 104, control 106.
|
|
recovery time, 20.0% lower, relative time 0.80, p = 0.19, treatment 105, control 106.
|
|
risk of no viral clearance, 3.8% lower, RR 0.96, p = 0.45, treatment 104, control 106, inverted to make RR<1 favor treatment, mid-recovery, day 7.
|
|
risk of no viral clearance, no change, RR 1.00, p = 1.00, treatment 104, control 106, inverted to make RR<1 favor treatment, day 14.
|
|
risk of no viral clearance, 2.9% lower, RR 0.97, p = 0.83, treatment 104, control 106, inverted to make RR<1 favor treatment, day 3.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Corada et al., 6 May 2024, Double Blind Randomized Controlled Trial, placebo-controlled, USA, peer-reviewed, 17 authors, average treatment delay 5.0 days.
Safety, Efficacy, and Pharmacokinetics of Combination SARS-CoV-2 Neutralizing Monoclonal Antibodies BMS-986414 (C135-LS) and BMS-986413 (C144-LS) Administered Subcutaneously in Non-Hospitalized Persons with COVID-19 in a Phase 2 Trial AUTHORS
doi:10.20411/pai.v9i1.660
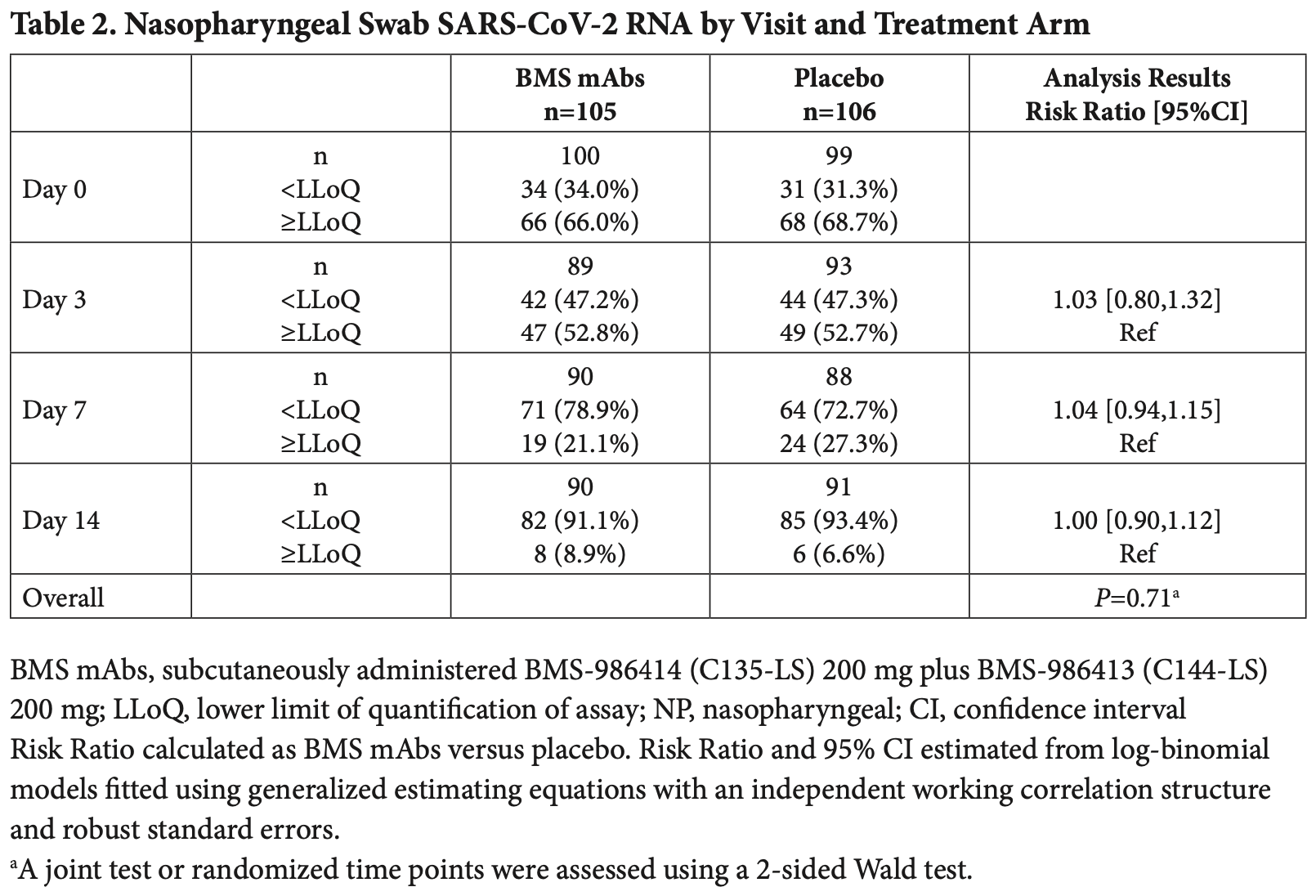
Background: Outpatient COVID-19 monoclonal antibody (mAb) treatment via subcutaneous delivery, if effective, overcomes the logistical burdens of intravenous administration. Methods: ACTIV-2/A5401 was a randomized, masked placebo-controlled platform trial where participants with COVID-19 at low risk for progression were randomized 1:1 to subcutaneously administered BMS-986414 (C135-LS) 200 mg, plus BMS-986413 (C144-LS) 200 mg, (BMS mAbs), or placebo. Coprimary outcomes were time to symptom improvement through 28 days; nasopharyngeal SARS-CoV-2 RNA below the lower limit of quantification (LLoQ) on days 3, 7, or 14; and treatment-emergent grade 3 or higher adverse events (TEAEs) through 28 days. Results: A total of 211 participants (105 BMS mAbs and 106 placebo) initiated study product. Time to symptom improvement favored the active therapy but was not significant (median 8 vs 10 days, P=0.19). There was no significant difference in the proportion with SARS-CoV-2 RNA <LLoQ at day 3 (risk ratio [RR] for BMS mAbs versus placebo: 1.03; 95%CI: 0.80, 1.32), at day 7 (RR: 1.04; 95%CI: 0.94, 1.15), or at day 14 (RR: 1.00; 95%CI: 0.90, 1.12). Fewer grade 3 TEAEs were reported for the BMS mAbs arm than placebo (RR: 0.58 [95%CI: 0.25, 1.32]). Through day 28, there were no deaths, and there were 4 hospitalizations in the BMS mAbs arm versus 3 in the placebo arm. Higher early plasma mAb concentrations were associated with more favorable outcomes. Conclusions: While safe, the BMS mAbs delivered subcutaneously were not effective at treating COVID-19 at low risk for progression. The lack of clinically significant activity may relate to the pharmacokinetics of subcutaneous administration of mAbs.
Sciences, Merck, and GSK/ViiV and research support through the institution from Gilead Sciences and GSK/ViiV. DAW has received funding to the institution to support research and honoraria for advisory boards and consulting from Gilead Sciences. JZL consulted for Abbvie. DMS has consulted for Fluxergy, Kiadis, Linear Therapies, Matrix BioMed, Arena Pharmaceuticals, VxBiosciences, Model Medicines, Bayer Pharmaceuticals, Signant Health, and Brio Clinical. JJE is an ad hoc consultant to GSK/VIR and data monitoring committee chair for Adagio Phase III studies. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
SUPPLEMENTARY DATA Supplementary materials are available at the Pathogens and Immunity website. Supplementary data may be provided by the authors to benefit the reader. Supplementary data are not copyedited and are the sole responsibility of the authors. Questions or comments related to supplementary materials should be addressed to the corresponding author.
Supplementary Tables and Figures
References
Belote, Reece, Robinson, Jensen, Carllee et al., Noninferiority of Subcutaneous Versus Intravenous Casirivimab/Imdevimab for Outpatient Treatment of SARS-CoV-2 in a Real-World Setting, Monoclon Antib Immunodiagn Immunother, doi:10.1089/mab.2022.0008
Bender Ignacio, Chew, Moser, Currier, Eron et al., Safety and Efficacy of Combined Tixagevimab and Cilgavimab Administered Intramuscularly or Intravenously in Nonhospitalized Patients With COVID-19: 2 Randomized Clinical Trials, JAMA Netw Open, doi:10.1001/jamanetworkopen.2023.10039
Chew, Moser, Daar, Wohl, Li et al., Bamlanivimab reduces nasopharyngeal SARS-CoV-2 RNA levels but not symptom duration in non-hospitalized adults with COVID-19: A Phase 2 Randomized Clinical Trial. medRxiv : the preprint server for health sciences, doi:10.1101/2021.12.17.21268009
Currier, Moser, Eron, Chew, Smith et al., ACTIV-2: A Platform Trial for the Evaluation of Novel Therapeutics for the Treatment of Early COVID-19 in Outpatients, J Infect Dis, doi:10.1093/infdis/jiad246
Evering, Chew, Giganti, Moser, Pinilla et al., Safety and Efficacy of Combination SARS-CoV-2 Neutralizing Monoclonal Antibodies Amubarvimab Plus Romlusevimab in Nonhospitalized Patients With COVID-19, Ann Intern Med, doi:10.7326/M22-3428
Garcia-Knight, Anglin, Tassetto, Lu, Zhang et al., Infectious viral shedding of SARS-CoV-2 Delta following vaccination: A longitudinal cohort study, PLoS Pathog, doi:10.1371/journal.ppat.1010802
Gupta, Gonzalez-Rojas, Juarez, Casal, Moya et al., Early Treatment for Covid-19 with SARS-CoV-2 Neutralizing Antibody Sotrovimab, New Eng J Med, doi:10.1056/NEJMoa2107934
Jilg, Chew, Giganti, Daar, Wohl et al., One Week of Oral Camostat Versus Placebo in Nonhospitalized Adults With Mild-to-Moderate Coronavirus Disease 2019: A Randomized Controlled Phase 2 Trial, Clin Infect Dis, doi:10.1093/cid/ciad342
Magyarics, Leslie, Bartko, Rouha, Luperchio et al., Randomized, Double-Blind, Placebo-Controlled, Single-Ascending-Dose Study of the Penetration of a Monoclonal Antibody Combination (ASN100) Targeting Staphylococcus aureus Cytotoxins in the Lung Epithelial Lining Fluid of Healthy Volunteers, Antimicrob Agents Chemother, doi:10.1128/AAC.00350-19
Mccreary, Bariola, Wadas, Shovel, Wisniewski et al., Association of Subcutaneous or Intravenous Administration of Casirivimab and Imdevimab Monoclonal Antibodies With Clinical Outcomes in Adults With COVID-19, JAMA Netw Open, doi:10.1001/jamanetworkopen.2022.6920
Moser, Chew, Giganti, Li, Aga et al., Statistical Challenges When Analyzing SARS-CoV-2 RNA Measurements Below the Assay Limit of Quantification in COVID-19 Clinical Trials, J Infect Dis, doi:10.1093/infdis/jiad285
Moser, Chew, Ritz, Newell, Javan et al., Pooling Different Placebos as a Control Group in a Randomized Platform Trial: Benefits and Challenges From Experience in the AC-TIV-2 COVID-19 Trial, J Infect Dis, doi:10.1093/infdis/jiad209
Robbiani, Gaebler, Muecksch, Lorenzi, Wang et al., Convergent antibody responses to SARS-CoV-2 in convalescent individuals, Nature, doi:10.1038/s41586-020-2456-9
Schaefer-Babajew, Wang, Muecksch, Cho, Loewe et al., Antibody feedback regulates immune memory after SARS-CoV-2 mRNA vaccination, Nature, doi:10.1038/s41586-022-05609-w
Taiwo, Chew, Moser, Wohl, Daar et al., Phase 2 Safety and Antiviral Activity of SAB-185, a Novel Polyclonal Antibody Therapy for Nonhospitalized Adults With COVID-19, J Infect Dis, doi:10.1093/infdis/jiad013
Tulledge-Scheitel, Bell, Larsen, Bierle, Takahashi et al., A mobile unit overcomes the challenges to monoclonal antibody infusion for COVID-19 in skilled care facilities, J Am Geriatr Soc, doi:10.1111/jgs.17090
Weinreich, Sivapalasingam, Norton, Ali, Gao et al., REGEN-COV Antibody Combination and Outcomes in Outpatients with Covid-19, New Eng J Med, doi:10.1056/NEJMoa2108163
Wiltz, Feehan, Molinari, Ladva, Truman et al., Racial and Ethnic Disparities in Receipt of Medications for Treatment of COVID-19 -United States, March 2020-August 2021, MMWR Morb Mortal Wkly Rep, doi:10.15585/mmwr.mm7103e1
Wood, Aleem, Davis, Providing Access To Monoclonal Antibody Treatment Of Coronavirus (COVID-19) Patients In Rural And Underserved Areas. StatPearls. Treasure Island
Wu, Guo, Yuan, Cao, Wang et al., Duration of viable virus shedding and polymerase chain reaction positivity of the SARS-CoV-2 Omicron variant in the upper respiratory tract: a systematic review and meta-analysis, Int J Infect Dis, doi:10.1016/j.ijid.2023.02.011
Wu, Kumar, Moore, Hall, Vysniauskaite et al., Disparities in COVID-19 Monoclonal Antibody Delivery: a Retrospective Cohort Study, J Gen Intern Med, doi:10.1007/s11606-022-07603-4