Feb 19 |
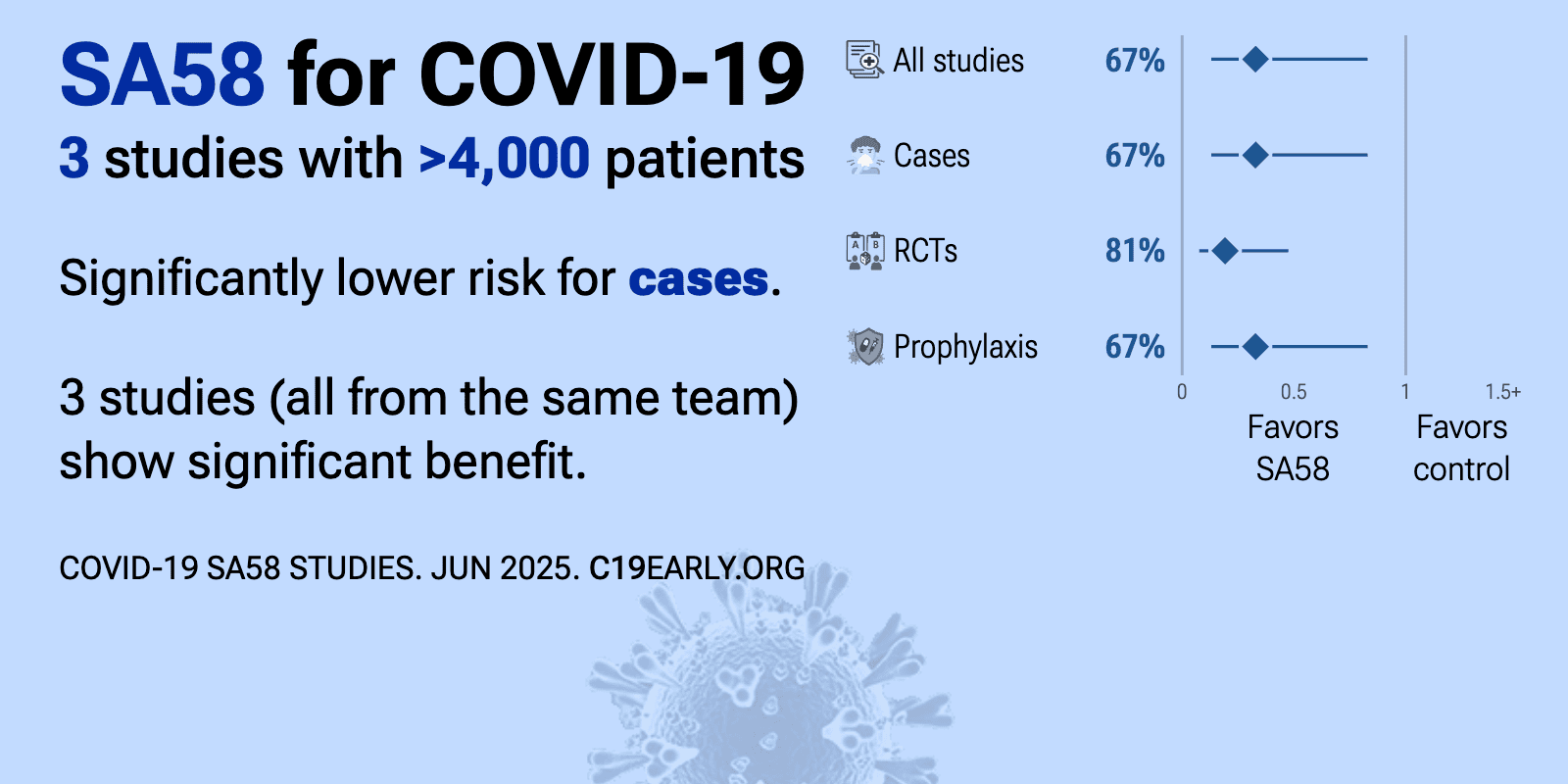
SA58 reduced COVID-19 risk: real-time meta-analysis of 3 studies | |
| Significantly lower risk is seen for cases. 3 studies (all from the same team/sponsor) show significant benefit. Meta-analysis using the most serious outcome reported shows 67% [17‑87%] lower risk. Results are better for Random.. | ||
Dec 31 2023 |
et al., China CDC Weekly, doi:10.46234/ccdcw2023.040 | Safety and Effectiveness of SA58 Nasal Spray Against COVID-19 Infection in Medical Personnel: An Open-Label, Blank-Controlled Study — Hohhot City, Inner Mongolia Autonomous Region, China, 2022 |
| 78% fewer cases (p=0.0001). Prospective study of 3,368 medical personnel in China showing significantly lower COVID-19 cases with SA58 nasal spray use. | ||
May 25 2023 |
et al., Emerging Microbes & Infections, doi:10.1080/22221751.2023.2212806 | Post-exposure prophylaxis with SA58 (anti-SARS-COV-2 monoclonal antibody) nasal spray for the prevention of symptomatic COVID-19 in healthy adult workers: a randomized, single-blind, placebo-controlled clinical study |
| 81% fewer symptomatic cases (p=0.0004) and 62% fewer cases (p=0.0001). RCT 1,222 healthy adult workers in China showing SA58 (anti-SARS-CoV-2 monoclonal antibody) nasal spray reduced symptomatic COVID-19 by 81% and SARS-COV-2 infection by 62% compared to placebo when used as post-exposure prophylaxis within .. | ||
Mar 20 2023 |
et al., medRxiv, doi:10.1101/2023.03.19.23287462 | Safety and Effectiveness of SA58 Nasal Spray against SARS-CoV-2 family transmission: an exploratory single-arm trial |
| 34% fewer cases (p<0.0001). Exploratory single-arm trial of 70 family contacts showing a protective effect of SA58 nasal spray against household SARS-CoV-2 transmission. The incidence of infection was 62.9% in the experimental group versus 94.8% in a contemporaneous.. | ||