Safety and Effectiveness of SA58 Nasal Spray Against COVID-19 Infection in Medical Personnel: An Open-Label, Blank-Controlled Study — Hohhot City, Inner Mongolia Autonomous Region, China, 2022
et al., China CDC Weekly, doi:10.46234/ccdcw2023.040, NCT05664919, Dec 2023
SA58 for COVID-19
55th treatment shown to reduce risk in
December 2023, now with p = 0.018 from 3 studies.
Efficacy is variant dependent.
Lower risk for cases.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
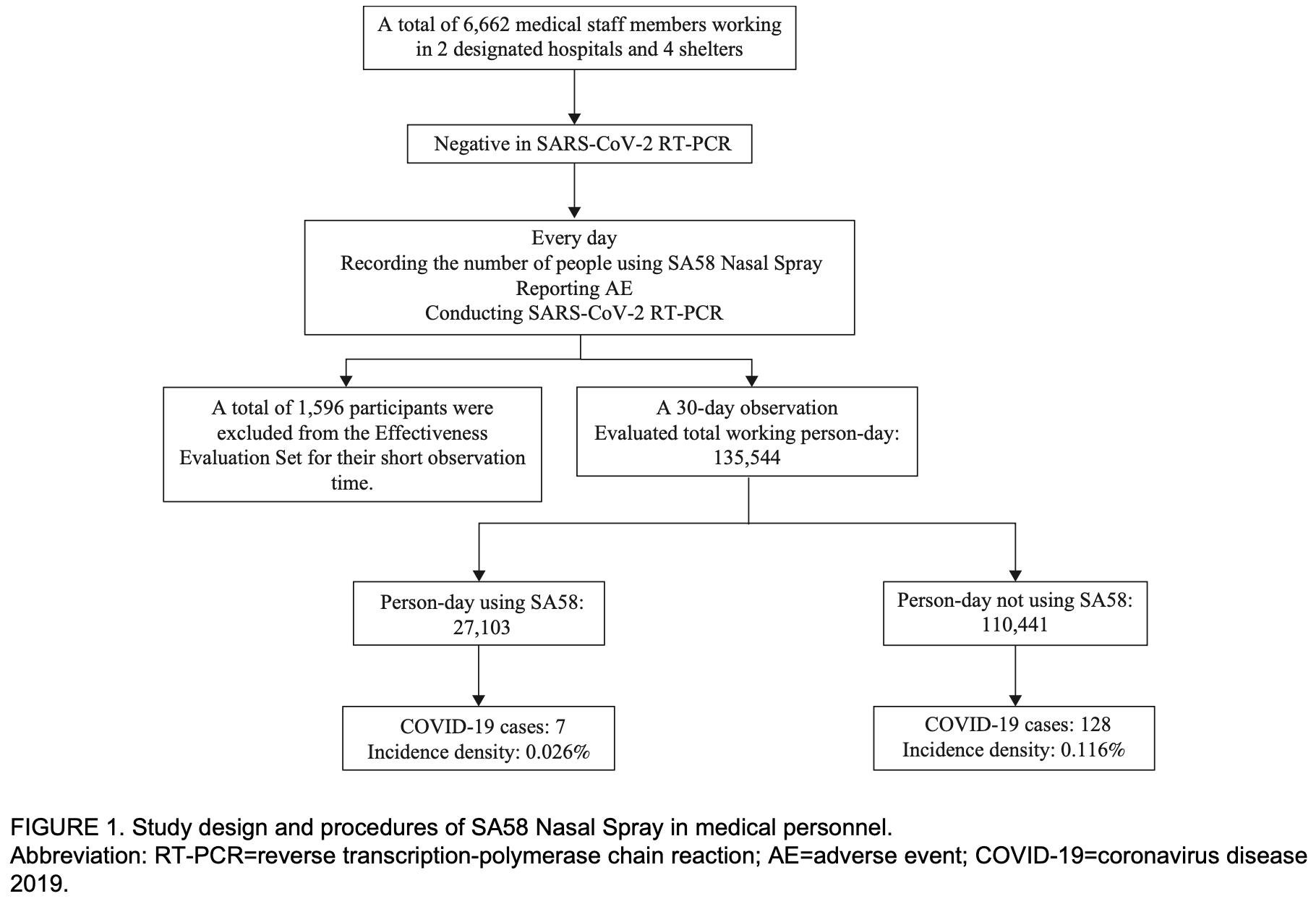
Prospective study of 3,368 medical personnel in China showing significantly lower COVID-19 cases with SA58 nasal spray use.
Targeted administration to the respiratory tract provides treatment directly
to the typical source of initial SARS-CoV-2 infection and replication, and
allows for rapid onset of action, higher local drug concentration, and reduced systemic side effects.
Standard of Care (SOC) for COVID-19 in the study country,
China, is average with moderate efficacy for approved treatments4.
|
risk of case, 77.7% lower, RR 0.22, p < 0.001, relative cases per person-day.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
1.
Si et al., Safety and Effectiveness of SA58 Nasal Spray Against COVID-19 Infection in Medical Personnel: An Open-Label, Blank-Controlled Study — Hohhot City, Inner Mongolia Autonomous Region, China, 2022, China CDC Weekly, doi:10.46234/ccdcw2023.040.
2.
Song et al., Post-exposure prophylaxis with SA58 (anti-SARS-COV-2 monoclonal antibody) nasal spray for the prevention of symptomatic COVID-19 in healthy adult workers: a randomized, single-blind, placebo-controlled clinical study, Emerging Microbes & Infections, doi:10.1080/22221751.2023.2212806.
Si et al., 31 Dec 2023, prospective, China, peer-reviewed, median age 34.0, 22 authors, study period 31 October, 2022 - 30 November, 2022, trial NCT05664919 (history).
Safety and Effectiveness of SA58 Nasal Spray Against COVID-19 Infection in Medical Personnel: An Open-Label, Blank-Controlled Study -Hohhot City, Inner Mongolia Autonomous Region, China, 2022
What is already known about this topic? The active ingredient of the SA58 Nasal Spray is a broad-spectrum neutralizing antibody with a high neutralizing capacity against different Omicron subvariants in vitro studies.
What is added by this report? This study demonstrated the safety and effectiveness of SA58 Nasal Spray against coronavirus disease 2019 (COVID-19) infection in medical personnel for the first time.
What are the implications for public health practice? This study provides an effective approach for the public to reduce their risk of COVID-19 infection. The findings of this research have the potential to significantly reduce the risk of infection and limit human-to-human transmission in the event of a COVID-19 outbreak.
References
Agrawal, Bedston, Mccowan, Oke, Patterson et al., Severe COVID-19 outcomes after full vaccination of primary schedule and initial boosters: pooled analysis of national prospective 8. cohort studies of 30 million individuals in England, Northern Ireland, Scotland, and Wales, Lancet, doi:10.1016/S0140-6736(22)01656-7
Cao, Jian, Zhang, Yisimayi, Hao et al., Rational identification of potent and broad sarbecovirus-neutralizing antibody cocktails from SARS convalescents, Cell Rep, doi:10.1016/j.celrep.2022.111845
Cao, Su, Guo, Sun, Deng et al., Potent neutralizing antibodies against SARS-CoV-2 identified by highthroughput single-cell sequencing of convalescent patients' B cells, Cell, doi:10.1016/j.cell.2020.05.025
Cao, Wang, Jian, Xiao, Song et al., Omicron escapes the majority of existing SARS-CoV-2 neutralizing antibodies, Nature, doi:10.1038/s41586-021-04385-3
Cao, Yisimayi, Jian, Song, Xiao et al., 2.12.1, BA.4 and BA.5 escape antibodies elicited by Omicron infection, Nature, doi:10.1038/s41586-022-04980-y
Castro Dopico, Ols, Loré, Hedestam, Immunity to SARS-CoV-2 induced by infection or vaccination, J Intern Med, doi:10.1111/joim.13372
Du, Cao, Zhu, Yu, Qi et al., Structurally resolved SARS-CoV-2 antibody shows high efficacy in severely infected hamsters and provides a potent cocktail pairing strategy, Cell, doi:10.1016/j.cell.2020.09.035
Mcmenamin, Lin, Wong, Cheung, Lau, Vaccine effectiveness of one, two, and three doses of BNT162b2 and CoronaVac against COVID-19 in Hong Kong: a population-based observational study, Lancet Infect Dis, doi:10.1016/S1473-3099(22)00345-0
Nyberg, Ferguson, Nash, Webster, Flaxman et al., Comparative analysis of the risks of hospitalisation and death associated with SARS-CoV-2 omicron (B. 1.1.529) and delta (B.1.617.2) variants in England: a cohort study, Lancet, doi:10.1016/S0140-6736(22)00462-7
DOI record:
{
"DOI": "10.46234/ccdcw2023.040",
"ISSN": [
"2096-7071"
],
"URL": "http://dx.doi.org/10.46234/ccdcw2023.040",
"author": [
{
"affiliation": [],
"family": "Si",
"given": "Shujie",
"sequence": "first"
},
{
"affiliation": [],
"name": "Pharmacy Department, Inner Mongolia Fourth Hospital, Hohhot City, Inner Mongolia Autonomous Region, China",
"sequence": "first"
},
{
"affiliation": [],
"family": "Jin",
"given": "Canrui",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Li",
"given": "Jianping",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Cao",
"given": "Yunlong",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kan",
"given": "Biao",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Xue",
"given": "Feng",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Sunney Xie",
"given": "Xiaoliang",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Fang",
"given": "Liang",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Zeng",
"given": "Gang",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Zhang",
"given": "Shuo",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hu",
"given": "Yaling",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Dong",
"given": "Xiaoping",
"sequence": "additional"
},
{
"affiliation": [],
"name": "Clinical Research and Development Department, Sinovac Biotech Co., Ltd., Beijing, China",
"sequence": "additional"
},
{
"affiliation": [],
"name": "Biomedical Pioneering Innovation Center (BIOPIC), Peking University, Beijing, China",
"sequence": "additional"
},
{
"affiliation": [],
"name": "Changping Laboratory, Beijing, China",
"sequence": "additional"
},
{
"affiliation": [],
"name": "State Key Laboratory for Infectious Disease Prevention and Control, NHC Key Laboratory of Medical Virology and Viral Diseases, National Institute for Viral Disease Control and Prevention, Chinese Center for Disease Control and Prevention, Beijing, China",
"sequence": "additional"
},
{
"affiliation": [],
"name": "Research and Development Department, Sinovac Life Sciences Co., Ltd., Beijing, China",
"sequence": "additional"
},
{
"affiliation": [],
"name": "Inner Mongolia Blood Center, Hohhot City, Inner Mongolia Autonomous Region, China",
"sequence": "additional"
},
{
"affiliation": [],
"name": "Center for Biosafety Mega-Science, Chinese Academy of Sciences, Wuhan City, Hubei Province, China",
"sequence": "additional"
},
{
"affiliation": [],
"name": "China Academy of Chinese Medical Sciences, Beijing, China",
"sequence": "additional"
},
{
"affiliation": [],
"name": "Shanghai Institute of Infectious Diseases and Biosafety, Shanghai, China",
"sequence": "additional"
}
],
"container-title": "China CDC Weekly",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2023,
3,
10
]
],
"date-time": "2023-03-10T14:19:05Z",
"timestamp": 1678457945000
},
"deposited": {
"date-parts": [
[
2023,
3,
10
]
],
"date-time": "2023-03-10T14:19:11Z",
"timestamp": 1678457951000
},
"indexed": {
"date-parts": [
[
2025,
5,
13
]
],
"date-time": "2025-05-13T13:10:12Z",
"timestamp": 1747141812915,
"version": "3.37.3"
},
"is-referenced-by-count": 5,
"issue": "10",
"issued": {
"date-parts": [
[
2023
]
]
},
"journal-issue": {
"issue": "10",
"published-print": {
"date-parts": [
[
2023
]
]
}
},
"language": "en",
"link": [
{
"URL": "https://weekly.chinacdc.cn/en/article/pdf/preview/10.46234/ccdcw2023.040",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "25413",
"original-title": [],
"page": "218-222",
"prefix": "10.46234",
"published": {
"date-parts": [
[
2023
]
]
},
"published-print": {
"date-parts": [
[
2023
]
]
},
"publisher": "Chinese Center for Disease Control and Prevention",
"reference": [
{
"DOI": "10.1111/joim.13372",
"doi-asserted-by": "crossref",
"key": "1",
"unstructured": "Castro Dopico X, Ols S, Loré K, Karlsson Hedestam GB. Immunity to SARS-CoV-2 induced by infection or vaccination. J Intern Med 2022;291(1):32 − 50. http://dx.doi.org/10.1111/joim.13372."
},
{
"DOI": "10.1038/s41586-022-04980-y",
"doi-asserted-by": "crossref",
"key": "2",
"unstructured": "Cao YL, Yisimayi A, Jian FC, Song WL, Xiao TH, Wang L, et al. BA. 2.12.1, BA.4 and BA.5 escape antibodies elicited by Omicron infection. Nature 2022;608(7923):593 − 602. http://dx.doi.org/10.1038/s41586-022-04980-y."
},
{
"DOI": "10.1038/s41586-021-04385-3",
"doi-asserted-by": "crossref",
"key": "3",
"unstructured": "Cao YL, Wang J, Jian FC, Xiao TH, Song WL, Yisimayi A, et al. Omicron escapes the majority of existing SARS-CoV-2 neutralizing antibodies. Nature 2022;602(7898):657 − 63. http://dx.doi.org/10.1038/s41586-021-04385-3."
},
{
"DOI": "10.1016/j.cell.2020.05.025",
"doi-asserted-by": "crossref",
"key": "4",
"unstructured": "Cao YL, Su B, Guo XH, Sun WJ, Deng YQ, Bao LL, et al. Potent neutralizing antibodies against SARS-CoV-2 identified by high-throughput single-cell sequencing of convalescent patients' B cells. Cell 2020;182(1):73 − 84.e16. http://dx.doi.org/10.1016/j.cell.2020.05.025."
},
{
"DOI": "10.1016/j.celrep.2022.111845",
"doi-asserted-by": "crossref",
"key": "5",
"unstructured": "Cao YL, Jian FC, Zhang ZY, Yisimayi A, Hao XH, Bao LL, et al. Rational identification of potent and broad sarbecovirus-neutralizing antibody cocktails from SARS convalescents. Cell Rep 2022;41(12):111845. http://dx.doi.org/10.1016/j.celrep.2022.111845."
},
{
"DOI": "10.1016/j.cell.2020.09.035",
"doi-asserted-by": "crossref",
"key": "6",
"unstructured": "Du S, Cao YL, Zhu QY, Yu P, Qi FF, Wang GP, et al. Structurally resolved SARS-CoV-2 antibody shows high efficacy in severely infected hamsters and provides a potent cocktail pairing strategy. Cell 2020;183(4):1013 − 23.e13. http://dx.doi.org/10.1016/j.cell.2020.09.035."
},
{
"DOI": "10.1016/S0140-6736(22)00462-7",
"doi-asserted-by": "crossref",
"key": "7",
"unstructured": "Nyberg T, Ferguson NM, Nash SG, Webster HH, Flaxman S, Andrews N, et al. Comparative analysis of the risks of hospitalisation and death associated with SARS-CoV-2 omicron (B. 1.1.529) and delta (B.1.617.2) variants in England: a cohort study. Lancet 2022;399(10332):1303 − 12. http://dx.doi.org/10.1016/S0140-6736(22)00462-7."
},
{
"DOI": "10.1016/S0140-6736(22)01656-7",
"doi-asserted-by": "crossref",
"key": "8",
"unstructured": "Agrawal U, Bedston S, McCowan C, Oke J, Patterson L, Robertson C, et al. Severe COVID-19 outcomes after full vaccination of primary schedule and initial boosters: pooled analysis of national prospective cohort studies of 30 million individuals in England, Northern Ireland, Scotland, and Wales. Lancet 2022;400(10360):1305 − 20. http://dx.doi.org/10.1016/S0140-6736(22)01656-7."
},
{
"DOI": "10.1016/S1473-3099(22)00345-0",
"doi-asserted-by": "crossref",
"key": "9",
"unstructured": "McMenamin ME, Nealon J, Lin Y, Wong JY, Cheung JK, Lau EHY, et al. Vaccine effectiveness of one, two, and three doses of BNT162b2 and CoronaVac against COVID-19 in Hong Kong: a population-based observational study. Lancet Infect Dis 2022;22(10):1435 − 43. http://dx.doi.org/10.1016/S1473-3099(22)00345-0."
}
],
"reference-count": 9,
"references-count": 9,
"relation": {},
"resource": {
"primary": {
"URL": "https://weekly.chinacdc.cn/en/article/doi/10.46234/ccdcw2023.040"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Safety and Effectiveness of SA58 Nasal Spray Against COVID-19 Infection in Medical Personnel: An Open-Label, Blank-Controlled Study — Hohhot City, Inner Mongolia Autonomous Region, China, 2022",
"type": "journal-article",
"volume": "5"
}
