Leritrelvir was adopted
in 1 country.
Mar 1 |
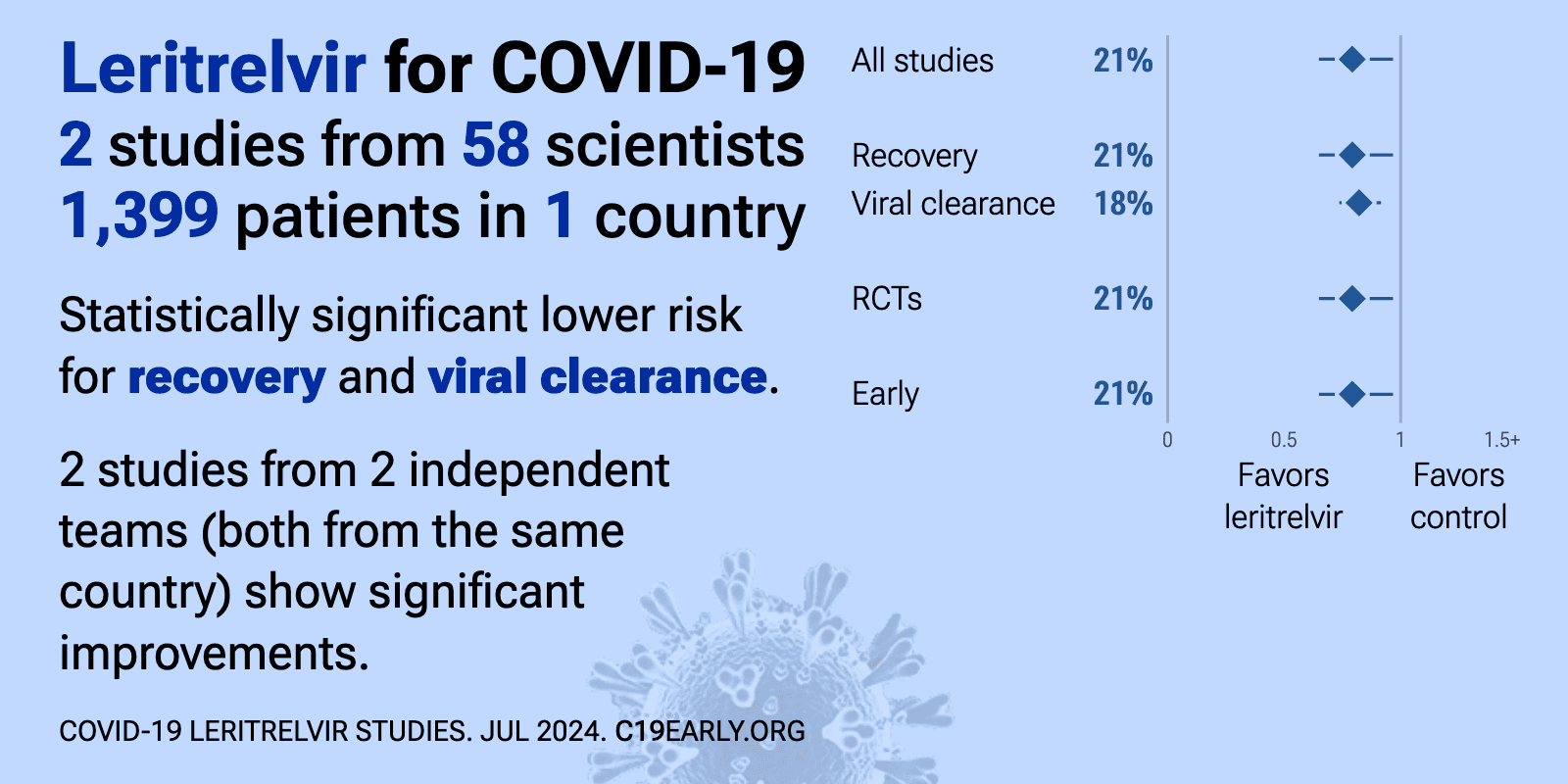
Leritrelvir for COVID-19: real-time meta-analysis of 2 studies | |
| Significantly lower risk is seen for recovery and viral clearance. 2 studies from 2 independent teams (both from the same country) show significant benefit. Meta-analysis using the most serious outcome reported shows 21% [3‑35%.. | ||
Jul 6 2024 |
et al., Quantitative Biology, doi:10.1002/qub2.60 | Assessing the inhibition efficacy of clinical drugs against the main proteases of SARS‐CoV‐2 variants and other coronaviruses |
| In vitro study showing that leritrelvir and GC376 remained effective against some nirmatrelvir- and ensitrelvir-resistant Mpro mutants. Leritrelvir showed better broad-spectrum activity against other pathogenic coronaviruses compared to e.. | ||
Mar 29 2024 |
et al., Nature Microbiology, doi:10.1038/s41564-024-01618-9 | Preclinical evaluation of the SARS-CoV-2 Mpro inhibitor RAY1216 shows improved pharmacokinetics compared with nirmatrelvir |
| In vitro and K18-hACE2 transgenic mouse study showing the preclinical efficacy of RAY1216, an inhibitor of the SARS-CoV-2 main protease (Mpro), against multiple SARS-CoV-2 variants. RAY1216 forms a covalent bond with the catalytic cystein.. | ||
Jan 31 2024 |
et al., eClinicalMedicine, doi:10.1016/j.eclinm.2023.102359 | Leritrelvir for the treatment of mild or moderate COVID-19 without co-administered ritonavir: a multicentre randomised, double-blind, placebo-controlled phase 3 trial |
| 17% improved recovery (p=0.002) and 16% improved viral clearance (p=0.01). RCT 1,359 COVID-19 outpatients showing faster recovery with leritrelvir monotherapy (without ritonavir), 251 vs. 271 hours, and improved viral clearance. There were no significant differences in adverse events between groups. | ||
Sep 30 2023 |
et al., eClinicalMedicine, doi:10.1016/j.eclinm.2023.102189 | Antiviral efficacy of RAY1216 monotherapy and combination therapy with ritonavir in patients with COVID-19: a phase 2, single centre, randomised, double-blind, placebo-controlled trial |
| 35% faster recovery (p=0.04) and 21% improved viral clearance (p=0.01). RCT 60 hospitalized COVID-19 patients in China showing imporoved recovery and viral clearance with RAY1216, a 3CLpro inhibitor. | ||