Feb 27 |
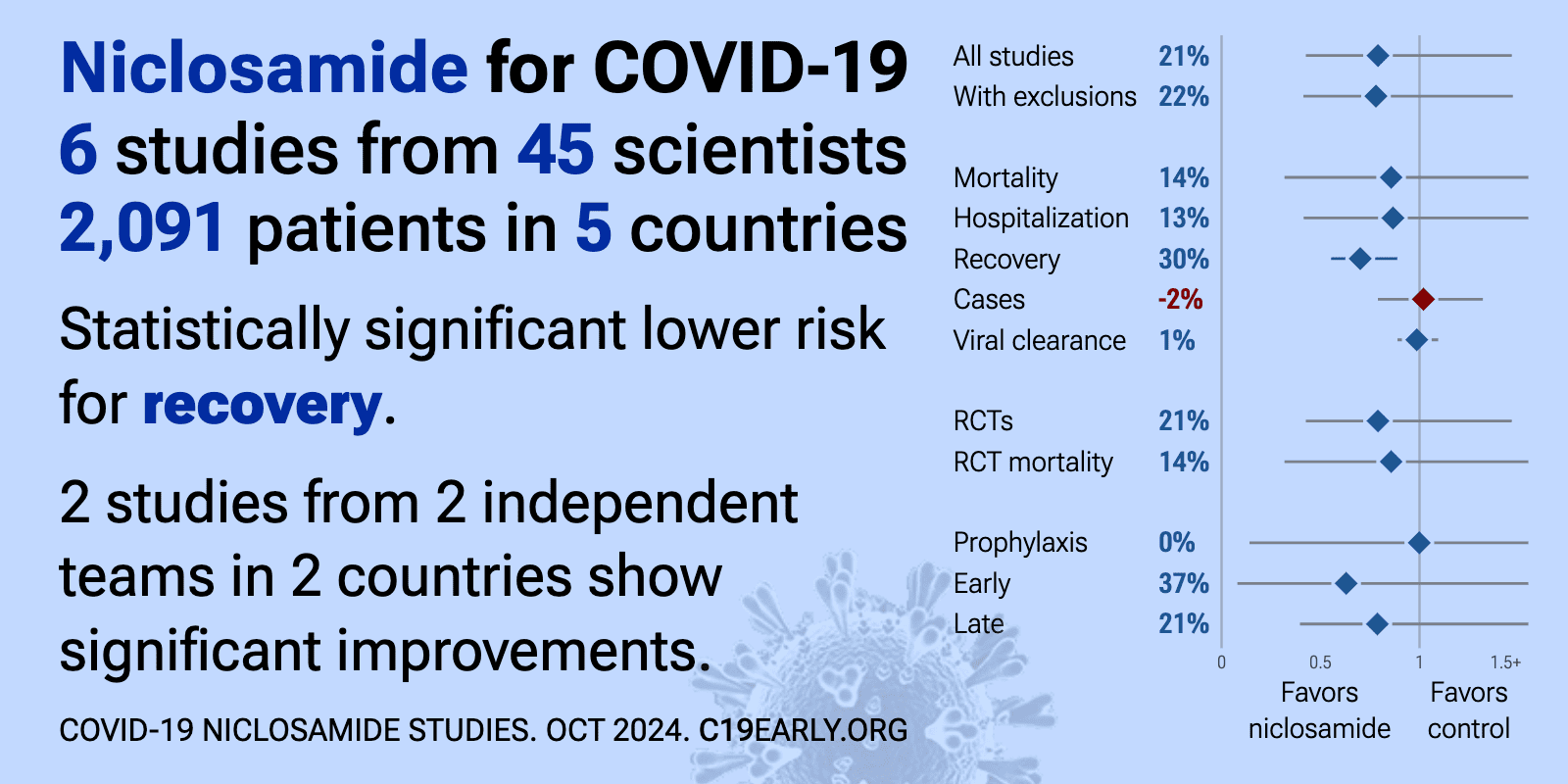
Niclosamide reduces COVID-19 risk: real-time meta-analysis of 7 studies (Version 7) | |
| Significantly lower risk is seen for recovery. 3 studies from 3 independent teams in 3 countries show significant benefit. Meta-analysis using the most serious outcome reported shows 28% [9‑43%] lower risk. Results are similar .. | ||
Aug 1 2025 |
et al., Nature Communications, doi:10.1038/s41467-025-62423-4 | A randomized, double-blind, placebo-controlled trial of niclosamide nanohybrid for the treatment of patients with mild to moderate COVID-19 |
| 29% faster recovery (p=0.008) and 56% improved viral clearance (p=0.16). RCT 300 patients with mild to moderate COVID-19 showing significant symptom improvement with niclosamide nanohybrid (CP-COV03). The high-dose group showed no significant benefit in time to symptom improvement in the primary analysis, whic.. | ||
Jun 12 2025 |
et al., Virology, doi:10.1016/j.virol.2025.110607 | SARS-CoV-2 host-pathogen interactome: insights into more players during pathogenesis |
| Review of SARS-CoV-2 host-pathogen interactions during viral pathogenesis, focusing on protein-protein interactions that facilitate viral entry, replication, immune evasion, assembly, and release. Authors comprehensively analyze how SARS-.. | ||
Apr 30 2025 |
et al., Health Technology Assessment, doi:10.3310/MTRS8833 | Lessons from the PROTECT-CH COVID-19 platform trial in care homes |
| Discussion of a planned COVID-19 platform trial (PROTECT-CH) in care homes that failed to start recruitment. The trial was designed to test prophylactic antiviral interventions (initially ciclesonide and niclosamide) to reduce SARS-CoV-2 .. | ||
Feb 12 2025 |
et al., Viruses, doi:10.3390/v17020252 | Inhaled Dry Powder of Antiviral Agents: A Promising Approach to Treating Respiratory Viral Pathogens |
| Review of inhaled dry powder antiviral formulations for treating respiratory viral infections, focusing on COVID-19. Authors explain that traditional antiviral tablets face limitations including systemic side effects and delayed onset of .. | ||
Nov 21 2024 |
et al., Discover Molecules, doi:10.1007/s44345-024-00005-5 | Exploring potential therapeutic candidates against COVID-19: a molecular docking study |
| In silico study showing potential inhibition of SARS-CoV-2 proteins by various compounds including dactinomycin, itraconazole, ivermectin, vitamin D, quercetin, curcumin, montelukast, bromhexine, hesperidin, EGCG and raloxifene. Authors p.. | ||
Aug 6 2024 |
et al., NCT04603924 | A Phase 2/3 Randomized and Placebo-Controlled Study of ANA001 in Moderate and Severe COVID-19 Patients |
| 30% lower mortality (p=1), 70% faster recovery (p=0.97), 57% higher hospital discharge (p=0.98), and no change in viral clearance (p=1). RCT 46 moderate to severe hospitalized COVID-19 patients showing shorter time to discharge and WHO clinical scale improvement with niclosamide, but no significant difference for resolution of all symptoms and viral clearance. | ||
Jun 19 2024 |
et al., Journal of Allergy and Clinical Immunology, doi:10.1016/j.jaci.2024.05.031 | Blockade of endolysosomal acidification suppresses TLR3-mediated pro-inflammatory signaling in airway epithelial cells |
| In vitro study showing that blocking endolysosomal acidification with bafilomycin A1, monensin, or niclosamide suppresses TLR3-mediated pro-inflammatory signaling in human small airway epithelial cells (HSAECs) stimulated with TLR3 agonis.. | ||
May 6 2024 |
et al., medRxiv, doi:10.1101/2024.05.06.24306928 | Clinical safety and pharmacokinetics of a novel oral niclosamide formulation compared with marketed niclosamide chewing tablets in healthy volunteers: a three-part randomized, double-blind, placebo-controlled trial |
| Phase 1 trial of 28 healthy volunteers showing an investigational niclosamide solution was reasonably well-tolerated up to 1,600mg for 7 days, with no severe adverse events. The most common adverse events were mild to moderate gastrointes.. | ||
Mar 29 2024 |
et al., Journal of Infection and Public Health, doi:10.1016/j.jiph.2024.03.030 | A Randomized Trial to Assess the Acceleration of Viral Clearance by the Combination Favipiravir/Ivermectin/Niclosamide in Mild-to-Moderate COVID-19 Adult Patients (FINCOV) |
| 39% improved recovery (p=0.19) and 6% improved viral clearance (p=0.75). RCT 60 low-risk outpatients, median age 31, with mild to moderate COVID-19 showing no significant differences with combined favipiravir/ivermectin/niclosamide treatment compared to favipiravir alone. There was limited room for improvement.. | ||
Mar 14 2024 |
et al., eClinicalMedicine, 10.1016/j.eclinm.2024.102517 | Early treatment with fluvoxamine, bromhexine, cyproheptadine, and niclosamide to prevent clinical deterioration in patients with symptomatic COVID-19: a randomized clinical trial |
| 98% lower ventilation (p<0.0001), 100% lower need for oxygen therapy (p<0.0001), 98% lower hospitalization (p<0.0001), and 59% lower long COVID (p<0.0001). RCT 995 outpatients showing significantly lower progression with early treatment within 48 hours using fluvoxamine, fluvoxamine+bromhexine, fluvoxamine+cyproheptadine, and niclosamide+bromhexine. 70% of patients received treatment within .. | ||
Mar 6 2024 |
et al., Nature Communications, doi:10.1038/s41467-024-46417-2 | SARS-CoV-2 virulence factor ORF3a blocks lysosome function by modulating TBC1D5-dependent Rab7 GTPase cycle |
| In vitro study showing that SARS-CoV-2 ORF3a protein hyperactivates the Rab7 GTPase to block lysosomal degradation and promote viral replication. Authors found that the chemical compound CID-1067700, a Rab7 inhibitor, reduced SARS-CoV-2 r.. | ||
Oct 11 2023 |
et al., Frontiers in Microbiology, doi:10.3389/fmicb.2023.1251065 | Niclosamide as a chemical probe for analyzing SARS-CoV-2 modulation of host cell lipid metabolism |
| In vitro study showing that niclosamide modulates host lipid metabolism and reduces infectious SARS-CoV-2 virion production in Vero E6 cells. Authors observed reorganization of the lipid profile in infected cells, with increased triglycer.. | ||
Sep 20 2023 |
et al., Journal of Clinical Medicine, doi:10.3390/jcm12186079 | SARS-CoV-2 Syncytium under the Radar: Molecular Insights of the Spike-Induced Syncytia and Potential Strategies to Limit SARS-CoV-2 Replication |
| Review of the molecular mechanisms of SARS-CoV-2 spike-induced syncytia formation and potential anti-fusogenic therapeutic strategies. The SARS-CoV-2 spike protein interacts with the ACE2 receptor on adjacent cells, triggering abnormal fu.. | ||
Jul 26 2023 |
et al., BMC Infectious Diseases, doi:10.1186/s12879-025-10584-4 (date from preprint) | Prophylaxis for renal patients at risk of COVID-19 infection: results from the intranasal niclosamide randomised, double blinded, placebo controlled arm of the PROTECT-V platform trial |
| 80% lower ventilation (p=0.25), 13% lower hospitalization (p=0.71), and 2% more symptomatic cases (p=0.89). RCT 1,651 patients with kidney disease showing no significant difference in symptomatic COVID-19, hospitalization, or mortality with intranasal niclosamide compared to placebo. The UNI911 nasal spray had very poor adherence and a higher w.. | ||
Jul 18 2023 |
et al., Biomedicines, doi:10.3390/biomedicines11072019 | Repurposing Niclosamide as a Novel Anti-SARS-CoV-2 Drug by Restricting Entry Protein CD147 |
| In vitro study showing that niclosamide reduces CD147 protein levels and inhibits SARS-CoV-2-induced upregulation of CD147 in A549-ACE2 cells. Authors find that the RNA-binding protein HuR binds to the 3'-UTR of BSG mRNA and upregulates C.. | ||
Jul 1 2023 |
et al., Acta Biochimica et Biophysica Sinica, doi:10.3724/abbs.2023129 | A pseudovirus-based method to dynamically mimic SARS-CoV-2-associated cell-to-cell fusion and transmission |
| In vitro study showing that niclosamide dramatically blocked the formation of syncytia mediated by SARS-CoV-2 spike protein pseudovirus-producing 293FT cells when cocultured with hACE2-expressing 293T cells at 1μM concentration. Authors d.. | ||
May 1 2023 |
, NCT04858425 | A 2-Part, 2-Arm, Phase 2, Randomized, Double-Blind, Placebo-Controlled Study on the Safety and Efficacy of Niclosamide in Patients With COVID-19 With Gastrointestinal Infection |
| 23% improved viral clearance (p=0.61). RCT 118 hospitalized patients with gastrointestinal infection showing no significant difference in viral clearance with niclosamide. Viral clearance results are available on clinicaltrials.gov but clinical results are missing. | ||
Jan 7 2023 |
et al., International Journal of Applied Pharmaceutics, doi:10.22159/ijap.2023v15i1.45850 | Niclosamide: a potential treatment option for COVID-19 |
| Review of niclosamide as a potential treatment option for COVID-19. Authors report antiviral activity against SARS-CoV-2 in vitro, with an IC50 of 0.28 μM. However, the currently available oral formulations limit systemic levels. To impro.. | ||
Dec 1 2022 |
et al., Journal of Pharmaceutical Technolgy, doi:10.37662/jpt.2022.999481 | Evaluation the plausibility of repurpose of levamisole and niclosamide in treatment of covid-19 |
| Review of the plausibility of repurposing levamisole and niclosamide for treatment of COVID-19. Niclosamide, an FDA-approved antihelminthic drug, has shown antiviral activity against various viruses including SARS-CoV, MERS-CoV, and poten.. | ||
Aug 9 2022 |
et al., Vaccines, doi:10.3390/vaccines10081284 | In Vitro Evaluation and Mitigation of Niclosamide’s Liabilities as a COVID-19 Treatment |
| In vitro study suggesting that niclosamide has variable potency against SARS-CoV-2 variants and may have cytotoxicity concerns and significant polypharmacology. Authors found that niclosamide inhibited SARS-CoV-2 infection in VeroE6 and H.. | ||
Jun 18 2022 |
et al., BMC Pharmacology and Toxicology, doi:10.1186/s40360-022-00580-8 (date from preprint) | Synergistic anti-SARS-CoV-2 activity of repurposed anti-parasitic drug combinations |
| In vitro study showing a strong synergistic effect of combinations of ivermectin, niclosamide, and chloroquine, with >10x reduction in IC50 compared to individual drugs. | ||
Apr 20 2022 |
et al., International Journal of Molecular Sciences, doi:10.3390/ijms23094576 | The Endolysosomal System: The Acid Test for SARS-CoV-2 |
| Review of the role of the endolysosomal system in SARS-CoV-2 infection. The endolysosomal system, which includes endosomes and lysosomes, is crucial for cellular homeostasis and host defense against pathogens. SARS-CoV-2 can enter cells b.. | ||
Apr 11 2022 |
et al., British Journal of Pharmacology, doi:10.1111/bph.15843 | Niclosamide—A promising treatment for COVID‐19 |
| Review of niclosamide as a promising treatment for COVID-19. Authors highlight niclosamide's potent antiviral activity against SARS-CoV-2 and its pleiotropic anti-inflammatory, antibacterial, bronchodilatory and anticancer effects demonst.. | ||
Feb 9 2022 |
et al., JAMA Network Open, doi:10.1001/jamanetworkopen.2021.44942 | Efficacy of Niclosamide vs Placebo in SARS-CoV-2 Respiratory Viral Clearance, Viral Shedding, and Duration of Symptoms Among Patients With Mild to Moderate COVID-19 |
| 18% faster recovery (p=0.28) and 24% improved viral clearance (p=0.45). RCT with 73 mild to moderate outpatients, showing faster recovery and improved viral clearance with niclosamide, without statistical significance. Greater improvements in recovery were seen for high-risk patients, again without statistica.. | ||
Dec 28 2021 |
, D., Pharmaceutical Research, doi:10.1007/s11095-021-03112-x | The pH Dependence of Niclosamide Solubility, Dissolution, and Morphology: Motivation for Potentially Universal Mucin-Penetrating Nasal and Throat Sprays for COVID19, its Variants and other Viral Infections |
| In vitro study showing that niclosamide has potential as a prophylactic nasal spray and early treatment throat spray for COVID-19 and other respiratory viral infections. Authors find that niclosamide's solubility and dissolution rate are.. | ||
Oct 18 2021 |
et al., Molecular Biology Reports, doi:10.1007/s11033-021-06770-7 | Niclosamide for Covid-19: bridging the gap |
| Review of niclosamide as a potential treatment for COVID-19. Authors highlight the anti-inflammatory and antiviral effects of niclosamide, which modulates the release of pro-inflammatory cytokines, inhibits the NF-κB/NLRP3 inflammasome an.. | ||
Sep 30 2021 |
et al., Annals of Medicine & Surgery, doi:10.1016/j.amsu.2021.102779 | A randomised controlled trial of effectiveness and safety of Niclosamide as add on therapy to the standard of care measures in COVID-19 management |
| 39% improved recovery (p=0.007). RCT with 75 COVID-19 patients showing significantly faster recovery but no change in mortality with niclosamide. The treatment group had more patients aged 60+ and more patients treated over a week after symptom onset. | ||
Jun 16 2021 |
et al., British Society For Nanomedicine Early Career Researcher Summer Meeting, 2021 | Niclosamide and ivermectin modulate caspase-1 activity and proinflammatory cytokine secretion in a monocytic cell line |
| In vitro study showing potential therapeutic effects of ivermectin and niclosamide on the immune system by reducing inflammation and modulating key proteins involved in the inflammatory response. Ivermectin and niclosamide reduced proinfl.. | ||
Feb 14 2021 |
et al., NCT04558021 | A Phase III, Randomized, Placebo-controlled, Clinical Trial to Evaluate the Efficacy and Safety of Co-administered Niclosamide in Patients Treated With an Established Regimen for Novel Coronavirus Infectious Disease (COVID-19) |
| Estimated 200 patient niclosamide late treatment RCT with results not reported over 5 years after estimated completion. | ||