A Randomized Trial to Assess the Acceleration of Viral Clearance by the Combination Favipiravir/Ivermectin/Niclosamide in Mild-to-Moderate COVID-19 Adult Patients (FINCOV)
et al., Journal of Infection and Public Health, doi:10.1016/j.jiph.2024.03.030, FINCOV, TCTR20230403007, Mar 2024
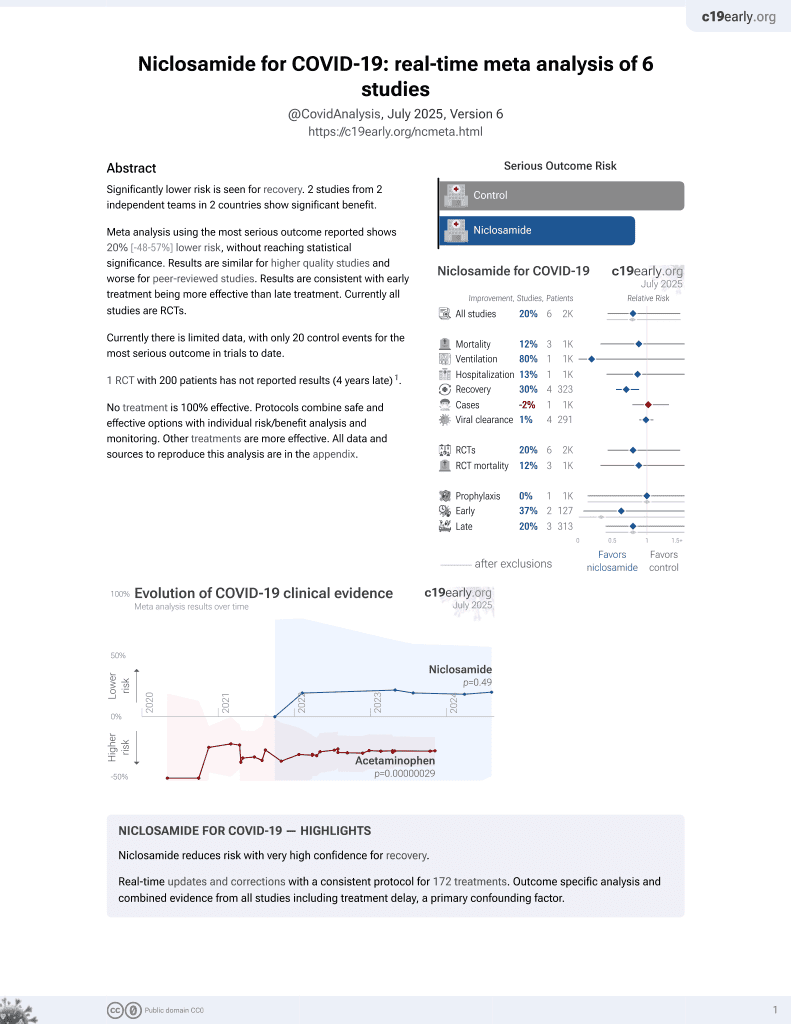
56th treatment shown to reduce risk in
August 2025, now with p = 0.0069 from 7 studies.
Lower risk for recovery.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
RCT 60 low-risk outpatients, median age 31, with mild to moderate COVID-19 showing no significant differences with combined favipiravir/ivermectin/niclosamide treatment compared to favipiravir alone. There was limited room for improvement with almost no progression and no hospitalization, ICU admission, supplemental oxygen, or mortality.
The combined group showed significantly improved visual analog scale (VAS) scores for cough, runny nose, and diarrhea from day 3.
Authors note that "the WHO-CPS were significantly decreased among FPV/IVM/NCL vs FPV alone on day 10", however the degree of improvement cannot be determined based on the values reported.
Authors state that "All data generated or analyzed during this study are included in this published article", which is incorrect - only summary statistics are published. The trial registration states that data will not be made available. This raises concerns, especially given many inconsistencies in the published data:
- E gene and ORF1 a/b gene day 1 Ct values are different between Table S1 and Table S3.
- Figure S1 shows 25% ≥ 38.5, however no number of 30 patients is 25%, and this does not match Table 2 (at most 23.3% ≥ 38.5).
- The first three calculations in section 3.3 all show p = 0.515, an unusual match for different calculations.
- "the FPV/IVM/NCL group had 0.62 cycles per day fewer than did the FPV group" does not match the data.
- Table 1 shows 30% loss of taste in the control group, however Table S2 shows a Q3 VAS score of 0, which is inconsistent.
- The abstract reference to significant differences for sore throat does not match the results in Table S6.
- Figure S1 shows 25% ≥ 38.5, however no number of 30 patients is 25%, and this does not match Table 2 (at most 23.3% ≥ 38.5).
- The first three calculations in section 3.3 all show p = 0.515, an unusual match for different calculations.
- "the FPV/IVM/NCL group had 0.62 cycles per day fewer than did the FPV group" does not match the data.
- Table 1 shows 30% loss of taste in the control group, however Table S2 shows a Q3 VAS score of 0, which is inconsistent.
- The abstract reference to significant differences for sore throat does not match the results in Table S6.
Not releasing data is a change from earlier COVID-19 trials by the same main author: TCTR20210615002 and TCTR20210609001 both indicate that individual patient level data would be available.
The trial was registered retrospectively. Discussion of prior research is very biased, however this may be required for publication. There were more patients with fever, anosmia, and loss of taste at baseline in the combined group. The adverse events reported show none of the expected side effects of ivermectin at the dose used.
This study is excluded in the after exclusion results of meta-analysis:
data consistency issues, very low risk patients/variants with almost no progression, all patients received known effective antiviral, baseline differences.
Study covers ivermectin and niclosamide.
|
risk of progression, no change, RR 1.00, p = 1.00, treatment 1 of 30 (3.3%), control 1 of 30 (3.3%), chest xray progression, day 6.
|
|
risk of progression, 66.7% lower, RR 0.33, p = 1.00, treatment 0 of 30 (0.0%), control 1 of 30 (3.3%), NNT 30, relative risk is not 0 because of continuity correction due to zero events (with reciprocal of the contrasting arm), chest xray progression, day 3.
|
|
risk of no recovery, 39.4% lower, RR 0.61, p = 0.19, treatment 30, control 30, combined symptoms.
|
|
risk of no recovery, 70.0% lower, RR 0.30, p = 0.048, treatment 30, control 30, mid-recovery, day 3, cough.
|
|
risk of no recovery, 14.3% lower, RR 0.86, p = 0.38, treatment 30, control 30, mid-recovery, day 3, sore throat.
|
|
risk of no recovery, 66.7% lower, RR 0.33, p = 0.41, treatment 30, control 30, inverted to make RR<1 favor treatment, mid-recovery, day 3, runny nose.
|
|
risk of no recovery, 50.0% lower, RR 0.50, p = 0.32, treatment 30, control 30, inverted to make RR<1 favor treatment, day 6, cough.
|
|
risk of no recovery, no change, RR 1.00, p = 0.61, treatment 30, control 30, WHO-CPS.
|
|
relative Ct improvement, 6.1% better, RR 0.94, p = 0.75, treatment median 9.15 IQR 9.7 n=30, control median 8.59 IQR 8.24 n=30, E gene, mid-recovery, day 5, primary outcome.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Siripongboonsitti et al., 29 Mar 2024, Randomized Controlled Trial, Thailand, peer-reviewed, 5 authors, study period 7 December, 2022 - 3 February, 2023, this trial uses multiple treatments in the treatment arm (combined with niclosamide) - results of individual treatments may vary, trial TCTR20230403007 (FINCOV).
A Randomized Trial to Assess the Acceleration of Viral Clearance by the Combination Favipiravir/Ivermectin/Niclosamide in Mild-to-Moderate COVID-19 Adult Patients (FINCOV)
doi:10.1016/j.jiph.2024.03.030
Background: The efficacy of the viral clearance and clinical outcomes of favipiravir (FPV) in outpatients being treated for coronavirus disease 2019 (COVID-19) is unclear. Ivermectin (IVM), niclosamide (NCL), and FPV demonstrated synergistic effects in vitro for exceed 78% inhibiting severe acute respiratory syndrome-coronavirus-2 (SARS-CoV-2) replication. Methods: A phase 2, open-label, 1:1, randomized, controlled trial was conducted on Thai patients with mild-to-moderate COVID-19 who received either combination FPV/IVM/NCL therapy or FPV alone to assess the rate of viral clearance among individuals with mild-to-moderate COVID-19. Results: Sixty non-high-risk comorbid patients with mild-to-moderate COVID-19 were randomized; 30 received FPV/IVM/NCL, and 30 received FPV alone. Mixed-effects multiple linear regression analysis of the cycle threshold value from SARS-CoV-2 PCR demonstrated no statistically significant differences in viral clearance rates between the combined FPV/IVM/NCL therapy group and the FPV-alone group. World Health Organization Clinical Progression scores and symptomatic improvement did not differ between arms on days 3, 6, and 10, and no adverse J o u r n a l P r e -p r o o f events were reported. No patients required hospitalization, intensive care unit admission, or supplemental oxygen or died within 28 days. C-reactive protein on day 3 was lower in the FPV/IVM/NCL group.
Conclusion: Viral clearance rates did not differ significantly between the FPV/IVM/NCL combination therapy and FPV-alone groups of individuals with mild-to-moderate COVID-19, although the combined regimen demonstrated a synergistic effect in vitro. No discernible clinical benefit was observed. Further research is required to explore the potential benefits of FVP beyond its antiviral effects.
Conflicts of Interest The authors declare that they have no competing interests.
J o u r n a l P r e -p r o o f
References
Abd-Elsalam, Noor, Badawi, Khalaf, Esmail et al., Clinical study evaluating the efficacy of ivermectin in COVID-19 treatment: A randomized controlled study, J Med Virol, doi:10.1002/jmv.27122
Abdulamir, Gorial, Saadi, Maulood, Hashim et al., A randomised controlled trial of effectiveness and safety of Niclosamide as add on therapy to the standard of care measures in COVID-19 management, Ann Med Surg (Lond), doi:10.1016/j.amsu.2021.102779
Audus, Knaub, Guillot, Schaeffer, The effect of protein binding on ivermectin uptake by bovine brain microvessel endothelial cells, Vet Res Commun, doi:10.1007/bf01839186
Backer, Sjöbring, Sonne, Weiss, Hostrup et al., A randomized, double-blind, placebo-controlled phase 1 trial of inhaled and intranasal niclosamide: A broad spectrum antiviral candidate for treatment of COVID-19, Lancet Reg Health Eur, doi:10.1016/j.lanepe.2021.100084
Bernal, Da Silva, Musungaie, Kovalchuk, Gonzalez et al., Molnupiravir for Oral Treatment of Covid-19 in Nonhospitalized Patients, N Engl J Med, doi:10.1056/NEJMoa2116044
Bhimraj, Morgan, Shumaker, Lavergne, Baden et al., Infectious Diseases Society of America Guidelines on the Treatment and Management of Patients with COVID-19, Clin Infect Dis, doi:10.1093/cid/ciaa478
Bramante, Huling, Tignanelli, Buse, Liebovitz et al., Randomized trial of metformin, ivermectin, and fluvoxamine for COVID-19, N Engl J Med, doi:10.1056/NEJMoa2201662
Buonfrate, Chesini, Martini, Roncaglioni, Fernandez et al., High-dose ivermectin for early treatment of COVID-19 (COVER study): a randomised, doubleblind, multicentre, phase II, dose-finding, proof-of-concept clinical trial, Int J Antimicrob Agents, doi:10.1016/j.ijantimicag.2021.106516
Cai, Yang, Liu, Chen, Shu et al., Experimental treatment with favipiravir for COVID-19: an open-label control study, Engineering, doi:10.1016/j.eng.2020.03.007.JournalPre-proof
Cairns, Dulko, Griffiths, Golan, Cohen et al., Efficacy of niclosamide vs placebo in sars-cov-2 respiratory viral clearance, viral shedding, and duration of symptoms among patients with mild to moderate COVID-19: A phase 2 randomized clinical trial, JAMA Netw Open, doi:10.1001/jamanetworkopen.2021.44942
Caly, Druce, Catton, Jans, Wagstaff, The FDA-approved drug ivermectin inhibits the replication of SARS-CoV-2 in vitro, Antiviral Res, doi:10.1016/j.antiviral.2020.104787
Chen, Huang, Cheng, Wu, Chen et al., Favipiravir versus arbidol for COVID-19: a randomized clinical trial, MedRxiv, doi:10.1101/2020.03.17.20037432
Doi, Ando, Kuwatsuka, Ishihara, Favipiravir, Favipiravir Observational Study Interim Report 3
Du, Chen, Favipiravir: pharmacokinetics and concerns about clinical trials for 2019-nCoV infection, Clin Pharmacol Ther, doi:10.1002/cpt.1844
Furuta, Komeno, Nakamura, Favipiravir (T-705), a broad spectrum inhibitor of viral RNA polymerase, Proc Jpn Acad, Ser B, doi:10.2183/pjab.93.027
Galan, Santos, Asato, Araújo, De Lima Moreira et al., Phase 2 randomized study on chloroquine, hydroxychloroquine or ivermectin in hospitalized patients with severe manifestations of SARS-CoV-2 infection, Pathog Glob Health, doi:10.1080/20477724.2021.1890887
Golan, Campos, Woolson, Cilla, Hanabergh et al., Favipiravir in patients with early mild-to-moderate COVID-19: a randomized controlled trial, Clin Infect Dis, doi:10.1093/cid/ciac712
Hammond, Leister-Tebbe, Gardner, Abreu, Wisemandle, Oral nirmatrelvir for high-risk, nonhospitalized adults with COVID-19, N Engl J Med, doi:10.1056/NEJMoa2118542
Hassanipour, Arab-Zozani, Amani, Heidarzad, Fathalipour et al., The efficacy and safety of Favipiravir in treatment of COVID-19: A systematic review and J o u r n a l P r e -p r o o f meta-analysis of clinical trials, Sci Rep, doi:10.1038/s41598-021-90551-6
Irie, Nakagawa, Fujita, Tamura, Eto et al., Population pharmacokinetics of favipiravir in patients with COVID-19, CPT Pharmacometrics Syst Pharmacol, doi:10.1002/psp4.12685
Ivashchenko, Dmitriev, Vostokova, Azarova, Blinow et al., AVIFAVIR for treatment of patients with moderate coronavirus disease 2019 (COVID-19): interim results of a phase II/III multicenter randomized clinical trial, Clin Infect Dis, doi:10.1093/cid/ciaa1176
Jeon, Ko, Lee, Choi, Byun et al., Identification of antiviral drug candidates against SARS-CoV-2 from FDA-approved drugs, Antimicrob Agents Chemother, doi:10.1128/aac.00819-20
Jitobaom, Boonarkart, Manopwisedjaroen, Punyadee, Borwornpinyo et al., Favipiravir and ivermectin show in vitro synergistic antiviral activity against SARS-CoV-2, Acta Virologica
Jitobaom, Boonarkart, Manopwisedjaroen, Punyadee, Borwornpinyo et al., Synergistic anti-SARS-CoV-2 activity of repurposed anti-parasitic drug combinations, BMC Pharmacol Toxicol, doi:10.1186/s40360-022-00580-8
Joshi, Parkar, Ansari, Vora, Talwar et al., Role of favipiravir in the treatment of COVID-19, Inter J Infect Dis, doi:10.1016/j.ijid.2020.10.069
Lim, Hor, Tay, Jelani, Tan et al., Efficacy of ivermectin treatment on disease progression among adults with mild to moderate COVID-19 and comorbidities: The I-TECH randomized clinical trial, JAMA Intern Med, doi:10.1001/jamainternmed.2022.0189
López-Medina, López, Hurtado, Dávalos, Ramirez et al., Effect of ivermectin on time to resolution of symptoms among adults with mild COVID-19: A randomized clinical trial, JAMA, doi:10.1001/jama.2021.3071.JournalPre-proof
Marshall, Murthy, Diaz, Adhikari, Angus et al., A minimal common outcome measure set for COVID-19 clinical research, Lancet Infect Dis, doi:10.1016/S1473-3099(20)30483-7
Mohan, Tiwari, Suri, Mittal, Patel et al., Single-dose oral ivermectin in mild and moderate COVID-19 (RIVET-COV): A single-centre randomized, placebo-controlled trial, J Infect Chemother, doi:10.1016/j.jiac.2021.08.021
Naggie, Boulware, Lindsell, Stewart, Gentile et al., Effect of ivermectin vs placebo on time to sustained recovery in outpatients with mild to moderate COVID-19: A randomized clinical trial, JAMA, doi:10.1001/jama.2022.18590
Naggie, Boulware, Lindsell, Stewart, Slandzicki et al., Effect of higher-dose ivermectin for 6 days vs placebo on time to sustained recovery in outpatients with COVID-19: A randomized clinical trial, JAMA, doi:10.1001/jama.2023.1650
Peña-Silva, Duffull, Steer, Jaramillo-Rincon, Gwee et al., Pharmacokinetic considerations on the repurposing of ivermectin for treatment of COVID-19, Br J Clin Pharmacol, doi:10.1111/bcp.14476
Ravikirti, Pattadar, Raj, Agarwal, Biswas, Evaluation of ivermectin as a potential treatment for mild to moderate COVID-19: A double-blind randomized placebo controlled trial in Eastern India, J Pharm Pharm Sci, doi:10.18433/jpps32105.JournalPre-proof
Reis, Silva, Silva, Thabane, Milagres et al., Effect of early treatment with ivermectin among patients with COVID-19, N Engl J Med, doi:10.1056/NEJMoa2115869
Shrestha, Budhathoki, Khadka, Shah, Pokharel et al., Favipiravir versus other antiviral or standard of care for COVID-19 treatment: a rapid systematic review and metaanalysis, Virol J, doi:10.1186/s12985-020-01412-z
Singh, Weiss, Goodman, Fisk, Kulkarni et al., Niclosamide-A promising treatment for COVID-19, Br J Pharmacol, doi:10.1111/bph.15843
Siripongboonsitti, Muadchimkaew, Tawinprai, Issaranon, Meepholkij et al., Favipiravir treatment in non-severe COVID-19: promising results from multicenter propensity score-matched study (FAVICOV), Sci Rep, doi:10.1038/s41598-023-42195-x
Siripongboonsitti, Tawinprai, Cheirsilpa, Ungtrakul, Krisorakun et al., The real-world clinical outcomes of favipiravir treatment with telemedicine monitoring in preventing disease progression in mild to moderate COVID-19 patients: a retrospective cohort study, Medicina, doi:10.3390/medicina59061098
Trivedi, Lee, Meibohm, Applications of pharmacometrics in the clinical development and pharmacotherapy of anti-infectives, Expert Rev Clin Pharmacol, doi:10.1586/ecp.13.6
Udwadia, Singh, Barkate, Patil, Rangwala et al., Efficacy and safety of favipiravir, an oral RNA-dependent RNA polymerase inhibitor, in mild-to-moderate COVID-19: A randomized, comparative, open-label, multicenter, phase 3 clinical trial, Inter J Infect Dis, doi:10.1016/j.ijid.2020.11.142
Vallejos, Zoni, Bangher, Villamandos, Bobadilla et al., Ivermectin to prevent hospitalizations in patients with COVID-19 (IVERCOR-COVID19) a randomized, double-blind, placebo-controlled trial, BMC Infect Dis, doi:10.1186/s12879-021-06348-5
Watson, Kissler, Day, Grad, White, Characterizing SARS-CoV-2 Viral Clearance Kinetics to Improve the Design of Antiviral Pharmacometric Studies, Antimicrob Agents Chemother, doi:10.1128/aac.00192-22
Watson, Kisslerc, Daya, Gradc, Whitea, Optimal design for phase 2 studies of SARS-CoV-2 antiviral drugs, MedRxiv
DOI record:
{
"DOI": "10.1016/j.jiph.2024.03.030",
"ISSN": [
"1876-0341"
],
"URL": "http://dx.doi.org/10.1016/j.jiph.2024.03.030",
"alternative-id": [
"S1876034124001060"
],
"assertion": [
{
"label": "This article is maintained by",
"name": "publisher",
"value": "Elsevier"
},
{
"label": "Article Title",
"name": "articletitle",
"value": "A randomized trial to assess the acceleration of viral clearance by the combination Favipiravir/Ivermectin/Niclosamide in mild-to-moderate COVID-19 adult patients (FINCOV)"
},
{
"label": "Journal Title",
"name": "journaltitle",
"value": "Journal of Infection and Public Health"
},
{
"label": "CrossRef DOI link to publisher maintained version",
"name": "articlelink",
"value": "https://doi.org/10.1016/j.jiph.2024.03.030"
},
{
"label": "Content Type",
"name": "content_type",
"value": "article"
},
{
"label": "Copyright",
"name": "copyright",
"value": "© 2024 The Author(s). Published by Elsevier Ltd on behalf of King Saud Bin Abdulaziz University for Health Sciences."
}
],
"author": [
{
"ORCID": "http://orcid.org/0000-0001-7256-9982",
"affiliation": [],
"authenticated-orcid": false,
"family": "Siripongboonsitti",
"given": "Taweegrit",
"sequence": "first"
},
{
"affiliation": [],
"family": "Tawinprai",
"given": "Kriangkrai",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Avirutnan",
"given": "Panisadee",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Jitobaom",
"given": "Kunlakanya",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-4745-4291",
"affiliation": [],
"authenticated-orcid": false,
"family": "Auewarakul",
"given": "Prasert",
"sequence": "additional"
}
],
"container-title": "Journal of Infection and Public Health",
"container-title-short": "Journal of Infection and Public Health",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"clinicalkey.fr",
"clinicalkey.jp",
"clinicalkey.es",
"clinicalkey.com.au",
"clinicalkey.com",
"elsevier.com",
"sciencedirect.com"
]
},
"created": {
"date-parts": [
[
2024,
3,
29
]
],
"date-time": "2024-03-29T07:40:22Z",
"timestamp": 1711698022000
},
"deposited": {
"date-parts": [
[
2024,
4,
2
]
],
"date-time": "2024-04-02T16:46:57Z",
"timestamp": 1712076417000
},
"funder": [
{
"DOI": "10.13039/501100016062",
"doi-asserted-by": "publisher",
"name": "National Health Foundation"
},
{
"DOI": "10.13039/100016175",
"doi-asserted-by": "publisher",
"name": "Chulabhorn Royal Academy"
}
],
"indexed": {
"date-parts": [
[
2024,
4,
3
]
],
"date-time": "2024-04-03T01:19:56Z",
"timestamp": 1712107196023
},
"is-referenced-by-count": 0,
"issue": "5",
"issued": {
"date-parts": [
[
2024,
5
]
]
},
"journal-issue": {
"issue": "5",
"published-print": {
"date-parts": [
[
2024,
5
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://www.elsevier.com/tdm/userlicense/1.0/",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2024,
5,
1
]
],
"date-time": "2024-05-01T00:00:00Z",
"timestamp": 1714521600000
}
},
{
"URL": "http://creativecommons.org/licenses/by-nc-nd/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2024,
3,
27
]
],
"date-time": "2024-03-27T00:00:00Z",
"timestamp": 1711497600000
}
}
],
"link": [
{
"URL": "https://api.elsevier.com/content/article/PII:S1876034124001060?httpAccept=text/xml",
"content-type": "text/xml",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://api.elsevier.com/content/article/PII:S1876034124001060?httpAccept=text/plain",
"content-type": "text/plain",
"content-version": "vor",
"intended-application": "text-mining"
}
],
"member": "78",
"original-title": [],
"page": "897-905",
"prefix": "10.1016",
"published": {
"date-parts": [
[
2024,
5
]
]
},
"published-print": {
"date-parts": [
[
2024,
5
]
]
},
"publisher": "Elsevier BV",
"reference": [
{
"DOI": "10.1056/NEJMoa2118542",
"article-title": "Oral nirmatrelvir for high-risk, nonhospitalized adults with COVID-19",
"author": "Hammond",
"doi-asserted-by": "crossref",
"first-page": "1397",
"issue": "15",
"journal-title": "N Engl J Med",
"key": "10.1016/j.jiph.2024.03.030_bib1",
"volume": "386",
"year": "2022"
},
{
"DOI": "10.1056/NEJMoa2116044",
"article-title": "Molnupiravir for oral treatment of Covid-19 in Nonhospitalized Patients",
"author": "Jayk Bernal",
"doi-asserted-by": "crossref",
"first-page": "509",
"issue": "6",
"journal-title": "N Engl J Med",
"key": "10.1016/j.jiph.2024.03.030_bib2",
"volume": "386",
"year": "2022"
},
{
"key": "10.1016/j.jiph.2024.03.030_bib3",
"unstructured": "U.S. Food and Drug Administration, FDA NEWS RELEASE, Coronavirus (COVID-19) Update: FDA Authorizes First Oral Antiviral for Treatment of COVID-19 2021 [updated December 22, 2021. Available from: 〈https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-first-oral-antiviral-treatment-covid-19〉]."
},
{
"DOI": "10.1002/cpt.1844",
"article-title": "Favipiravir: pharmacokinetics and concerns about clinical trials for 2019‐nCoV infection",
"author": "Du",
"doi-asserted-by": "crossref",
"first-page": "242",
"issue": "2",
"journal-title": "Clin Pharm Ther",
"key": "10.1016/j.jiph.2024.03.030_bib4",
"volume": "108",
"year": "2020"
},
{
"DOI": "10.2183/pjab.93.027",
"article-title": "Favipiravir (T-705), a broad spectrum inhibitor of viral RNA polymerase",
"author": "Furuta",
"doi-asserted-by": "crossref",
"first-page": "449",
"issue": "7",
"journal-title": "Proc Jpn Acad, Ser B",
"key": "10.1016/j.jiph.2024.03.030_bib5",
"volume": "93",
"year": "2017"
},
{
"DOI": "10.1016/j.eng.2020.03.007",
"article-title": "Experimental treatment with favipiravir for COVID-19: an open-label control study",
"author": "Cai",
"doi-asserted-by": "crossref",
"first-page": "1192",
"issue": "10",
"journal-title": "Engineering",
"key": "10.1016/j.jiph.2024.03.030_bib6",
"volume": "6",
"year": "2020"
},
{
"article-title": "Favipiravir versus arbidol for COVID-19: a randomized clinical trial",
"author": "Chen",
"journal-title": "MedRxiv",
"key": "10.1016/j.jiph.2024.03.030_bib7",
"year": "2020"
},
{
"article-title": "AVIFAVIR for treatment of patients with moderate coronavirus disease 2019 (COVID-19): interim results of a phase II/III multicenter randomized clinical trial",
"author": "Ivashchenko",
"journal-title": "Clin Infect Dis",
"key": "10.1016/j.jiph.2024.03.030_bib8",
"year": "2020"
},
{
"key": "10.1016/j.jiph.2024.03.030_bib9",
"unstructured": "Department of Medical Service. Clinical Practice Guideline to Diagnosis, Treatment and Prevention COVID-19 for Physician and Health Care Provider, 21 July 2021 [Available from: https://covid19.dms.go.th/Content/Select_Landding_page?contentId=139]."
},
{
"article-title": "Role of favipiravir in the treatment of COVID-19",
"author": "Joshi",
"journal-title": "Inter J Infect Dis",
"key": "10.1016/j.jiph.2024.03.030_bib10",
"year": "2020"
},
{
"article-title": "Fujita Health University",
"author": "Doi",
"journal-title": "Favipiravir Obs Study Interim Rep",
"key": "10.1016/j.jiph.2024.03.030_bib11",
"volume": "3",
"year": "2021"
},
{
"DOI": "10.1016/j.ijid.2020.11.142",
"article-title": "Efficacy and safety of favipiravir, an oral RNA-dependent RNA polymerase inhibitor, in mild-to-moderate COVID-19: A randomized, comparative, open-label, multicenter, phase 3 clinical trial",
"author": "Udwadia",
"doi-asserted-by": "crossref",
"first-page": "62",
"journal-title": "Inter J Infect Dis",
"key": "10.1016/j.jiph.2024.03.030_bib12",
"volume": "103",
"year": "2021"
},
{
"article-title": "The real-world clinical outcomes of favipiravir treatment with telemedicine monitoring in preventing disease progression in mild to moderate COVID-19 patients: a retrospective cohort study",
"author": "Siripongboonsitti",
"issue": "6",
"journal-title": "Med (Kaunas)",
"key": "10.1016/j.jiph.2024.03.030_bib13",
"volume": "59",
"year": "2023"
},
{
"DOI": "10.1038/s41598-023-42195-x",
"article-title": "Favipiravir treatment in non-severe COVID-19: promising results from multicenter propensity score-matched study (FAVICOV)",
"author": "Siripongboonsitti",
"doi-asserted-by": "crossref",
"issue": "1",
"journal-title": "Sci Rep",
"key": "10.1016/j.jiph.2024.03.030_bib14",
"volume": "13",
"year": "2023"
},
{
"DOI": "10.1038/s41598-021-90551-6",
"article-title": "The efficacy and safety of Favipiravir in treatment of COVID-19: A systematic review and meta-analysis of clinical trials",
"author": "Hassanipour",
"doi-asserted-by": "crossref",
"first-page": "1",
"issue": "1",
"journal-title": "Sci Rep",
"key": "10.1016/j.jiph.2024.03.030_bib15",
"volume": "11",
"year": "2021"
},
{
"DOI": "10.1186/s12985-020-01412-z",
"article-title": "Favipiravir versus other antiviral or standard of care for COVID-19 treatment: a rapid systematic review and meta-analysis",
"author": "Shrestha",
"doi-asserted-by": "crossref",
"first-page": "1",
"issue": "1",
"journal-title": "Virol J",
"key": "10.1016/j.jiph.2024.03.030_bib16",
"volume": "17",
"year": "2020"
},
{
"article-title": "Favipiravir in patients with early mild-to-moderate COVID-19: a randomized controlled trial",
"author": "Golan",
"journal-title": "Clin Infect Dis",
"key": "10.1016/j.jiph.2024.03.030_bib17",
"year": "2022"
},
{
"DOI": "10.1002/psp4.12685",
"article-title": "Population pharmacokinetics of favipiravir in patients with COVID-19",
"author": "Irie",
"doi-asserted-by": "crossref",
"first-page": "1161",
"issue": "10",
"journal-title": "CPT Pharmacomet Syst Pharm",
"key": "10.1016/j.jiph.2024.03.030_bib18",
"volume": "10",
"year": "2021"
},
{
"DOI": "10.1016/j.antiviral.2020.104787",
"article-title": "The FDA-approved drug ivermectin inhibits the replication of SARS-CoV-2 in vitro",
"author": "Caly",
"doi-asserted-by": "crossref",
"journal-title": "Antivir Res",
"key": "10.1016/j.jiph.2024.03.030_bib19",
"volume": "178",
"year": "2020"
},
{
"DOI": "10.1056/NEJMoa2115869",
"article-title": "Effect of early treatment with ivermectin among patients with COVID-19",
"author": "Reis",
"doi-asserted-by": "crossref",
"first-page": "1721",
"issue": "18",
"journal-title": "N Engl J Med",
"key": "10.1016/j.jiph.2024.03.030_bib20",
"volume": "386",
"year": "2022"
},
{
"DOI": "10.1016/j.ijantimicag.2021.106516",
"article-title": "High-dose ivermectin for early treatment of COVID-19 (COVER study): a randomised, double-blind, multicentre, phase II, dose-finding, proof-of-concept clinical trial",
"author": "Buonfrate",
"doi-asserted-by": "crossref",
"issue": "2",
"journal-title": "Int J Antimicrob Agents",
"key": "10.1016/j.jiph.2024.03.030_bib21",
"volume": "59",
"year": "2022"
},
{
"DOI": "10.1016/j.jiac.2021.08.021",
"article-title": "Single-dose oral ivermectin in mild and moderate COVID-19 (RIVET-COV): a single-centre randomized, placebo-controlled trial",
"author": "Mohan",
"doi-asserted-by": "crossref",
"first-page": "1743",
"issue": "12",
"journal-title": "J Infect Chemother",
"key": "10.1016/j.jiph.2024.03.030_bib22",
"volume": "27",
"year": "2021"
},
{
"DOI": "10.18433/jpps32105",
"article-title": "Evaluation of ivermectin as a potential treatment for mild to moderate COVID-19: a double-blind randomized placebo controlled trial in Eastern India",
"author": "Ravikirti",
"doi-asserted-by": "crossref",
"first-page": "343",
"journal-title": "J Pharm Pharm Sci",
"key": "10.1016/j.jiph.2024.03.030_bib23",
"volume": "24",
"year": "2021"
},
{
"DOI": "10.1001/jama.2023.1650",
"article-title": "Effect of higher-dose ivermectin for 6 days vs placebo on time to sustained recovery in outpatients with COVID-19: A randomized clinical trial",
"author": "Naggie",
"doi-asserted-by": "crossref",
"first-page": "888",
"issue": "11",
"journal-title": "JAMA",
"key": "10.1016/j.jiph.2024.03.030_bib24",
"volume": "329",
"year": "2023"
},
{
"DOI": "10.1001/jama.2022.18590",
"article-title": "Effect of ivermectin vs placebo on time to sustained recovery in outpatients with mild to moderate COVID-19: A randomized clinical trial",
"author": "Naggie",
"doi-asserted-by": "crossref",
"first-page": "1595",
"issue": "16",
"journal-title": "JAMA",
"key": "10.1016/j.jiph.2024.03.030_bib25",
"volume": "328",
"year": "2022"
},
{
"DOI": "10.1080/20477724.2021.1890887",
"article-title": "Phase 2 randomized study on chloroquine, hydroxychloroquine or ivermectin in hospitalized patients with severe manifestations of SARS-CoV-2 infection",
"author": "Galan",
"doi-asserted-by": "crossref",
"first-page": "235",
"issue": "4",
"journal-title": "Pathog Glob Health",
"key": "10.1016/j.jiph.2024.03.030_bib26",
"volume": "115",
"year": "2021"
},
{
"DOI": "10.1002/jmv.27122",
"article-title": "Clinical study evaluating the efficacy of ivermectin in COVID-19 treatment: a randomized controlled study",
"author": "Abd-Elsalam",
"doi-asserted-by": "crossref",
"first-page": "5833",
"issue": "10",
"journal-title": "J Med Virol",
"key": "10.1016/j.jiph.2024.03.030_bib27",
"volume": "93",
"year": "2021"
},
{
"DOI": "10.1001/jamainternmed.2022.0189",
"article-title": "Efficacy of ivermectin treatment on disease progression among adults with mild to moderate COVID-19 and comorbidities: The I-TECH randomized clinical trial",
"author": "Lim",
"doi-asserted-by": "crossref",
"first-page": "426",
"issue": "4",
"journal-title": "JAMA Intern Med",
"key": "10.1016/j.jiph.2024.03.030_bib28",
"volume": "182",
"year": "2022"
},
{
"DOI": "10.1186/s12879-021-06348-5",
"article-title": "Ivermectin to prevent hospitalizations in patients with COVID-19 (IVERCOR-COVID19) a randomized, double-blind, placebo-controlled trial",
"author": "Vallejos",
"doi-asserted-by": "crossref",
"first-page": "635",
"issue": "1",
"journal-title": "BMC Infect Dis",
"key": "10.1016/j.jiph.2024.03.030_bib29",
"volume": "21",
"year": "2021"
},
{
"DOI": "10.1001/jama.2021.3071",
"article-title": "Effect of ivermectin on time to resolution of symptoms among adults with mild COVID-19: a randomized clinical trial",
"author": "López-Medina",
"doi-asserted-by": "crossref",
"first-page": "1426",
"issue": "14",
"journal-title": "JAMA",
"key": "10.1016/j.jiph.2024.03.030_bib30",
"volume": "325",
"year": "2021"
},
{
"DOI": "10.1056/NEJMoa2201662",
"article-title": "Randomized trial of metformin, ivermectin, and fluvoxamine for COVID-19",
"author": "Bramante",
"doi-asserted-by": "crossref",
"first-page": "599",
"issue": "7",
"journal-title": "N Engl J Med",
"key": "10.1016/j.jiph.2024.03.030_bib31",
"volume": "387",
"year": "2022"
},
{
"DOI": "10.1111/bph.15843",
"article-title": "Niclosamide-a promising treatment for COVID-19",
"author": "Singh",
"doi-asserted-by": "crossref",
"first-page": "3250",
"issue": "13",
"journal-title": "Br J Pharm",
"key": "10.1016/j.jiph.2024.03.030_bib32",
"volume": "179",
"year": "2022"
},
{
"DOI": "10.1128/AAC.00819-20",
"article-title": "Identification of antiviral drug candidates against SARS-CoV-2 from FDA-approved drugs",
"author": "Jeon",
"doi-asserted-by": "crossref",
"issue": "7",
"journal-title": "Antimicrob Agents Chemother",
"key": "10.1016/j.jiph.2024.03.030_bib33",
"volume": "64",
"year": "2020"
},
{
"DOI": "10.1001/jamanetworkopen.2021.44942",
"article-title": "Efficacy of niclosamide vs placebo in sars-cov-2 respiratory viral clearance, viral shedding, and duration of symptoms among patients with mild to moderate COVID-19: A phase 2 randomized clinical trial",
"author": "Cairns",
"doi-asserted-by": "crossref",
"issue": "2",
"journal-title": "JAMA Netw Open",
"key": "10.1016/j.jiph.2024.03.030_bib34",
"volume": "5",
"year": "2022"
},
{
"article-title": "A randomised controlled trial of effectiveness and safety of Niclosamide as add on therapy to the standard of care measures in COVID-19 management",
"author": "Abdulamir",
"journal-title": "Ann Med Surg (Lond)",
"key": "10.1016/j.jiph.2024.03.030_bib35",
"volume": "69",
"year": "2021"
},
{
"DOI": "10.1186/s40360-022-00580-8",
"article-title": "Synergistic anti-SARS-CoV-2 activity of repurposed anti-parasitic drug combinations",
"author": "Jitobaom",
"doi-asserted-by": "crossref",
"first-page": "41",
"issue": "1",
"journal-title": "BMC Pharm Toxicol",
"key": "10.1016/j.jiph.2024.03.030_bib36",
"volume": "23",
"year": "2022"
},
{
"DOI": "10.3389/av.2023.12265",
"article-title": "Favipiravir and ivermectin show in vitro synergistic antiviral activity against SARS-CoV-2",
"author": "Jitobaom",
"doi-asserted-by": "crossref",
"journal-title": "Acta Virol",
"key": "10.1016/j.jiph.2024.03.030_bib37",
"volume": "67",
"year": "2023"
},
{
"key": "10.1016/j.jiph.2024.03.030_bib38",
"unstructured": "European Medicines Agency, ICH: E6 (R2) Good Clinical Practice–Scientific Guideline. 2016."
},
{
"key": "10.1016/j.jiph.2024.03.030_bib39",
"unstructured": "WHO, Health in all policies: Helsinki statement. Framework for country action. Health in all Policies Helsinki Statement Framework for Country Action. 2014."
},
{
"DOI": "10.1016/S1473-3099(20)30483-7",
"article-title": "A minimal common outcome measure set for COVID-19 clinical research",
"author": "Marshall",
"doi-asserted-by": "crossref",
"first-page": "e192",
"issue": "8",
"journal-title": "Lancet Infect Dis",
"key": "10.1016/j.jiph.2024.03.030_bib40",
"volume": "20",
"year": "2020"
},
{
"key": "10.1016/j.jiph.2024.03.030_bib41",
"unstructured": "COVID-19 Treatment Guidelines Panel, Coronavirus Disease 2019 (COVID-19) Treatment Guidelines 2020 [19 November 2022]. Available from: 〈https://www.covid19treatmentguidelines.nih.gov/〉."
},
{
"article-title": "Infectious diseases society of america guidelines on the treatment and management of patients with COVID-19",
"author": "Bhimraj",
"journal-title": "Clin Infect Dis",
"key": "10.1016/j.jiph.2024.03.030_bib42",
"year": "2020"
},
{
"DOI": "10.1128/aac.00192-22",
"article-title": "Characterizing SARS-CoV-2 viral clearance kinetics to improve the design of antiviral pharmacometric studies",
"author": "Watson",
"doi-asserted-by": "crossref",
"issue": "7",
"journal-title": "Antimicrob Agents Chemother",
"key": "10.1016/j.jiph.2024.03.030_bib43",
"volume": "66",
"year": "2022"
},
{
"DOI": "10.1586/ecp.13.6",
"article-title": "Applications of pharmacometrics in the clinical development and pharmacotherapy of anti-infectives",
"author": "Trivedi",
"doi-asserted-by": "crossref",
"first-page": "159",
"issue": "2",
"journal-title": "Expert Rev Clin Pharm",
"key": "10.1016/j.jiph.2024.03.030_bib44",
"volume": "6",
"year": "2013"
},
{
"key": "10.1016/j.jiph.2024.03.030_bib45",
"unstructured": "Watson J.A., Kisslerc S., Daya N.P., Gradc Y., Whitea N.J. Optimal design for phase 2 studies of SARS-CoV-2 antiviral drugs. MedRxiv 2021."
},
{
"DOI": "10.1111/bcp.14476",
"article-title": "Pharmacokinetic considerations on the repurposing of ivermectin for treatment of COVID-19",
"author": "Peña-Silva",
"doi-asserted-by": "crossref",
"first-page": "1589",
"issue": "3",
"journal-title": "Br J Clin Pharm",
"key": "10.1016/j.jiph.2024.03.030_bib46",
"volume": "87",
"year": "2021"
},
{
"DOI": "10.1007/BF01839186",
"article-title": "The effect of protein binding on ivermectin uptake by bovine brain microvessel endothelial cells",
"author": "Audus",
"doi-asserted-by": "crossref",
"first-page": "365",
"issue": "5",
"journal-title": "Vet Res Commun",
"key": "10.1016/j.jiph.2024.03.030_bib47",
"volume": "16",
"year": "1992"
},
{
"article-title": "A randomized, double-blind, placebo-controlled phase 1 trial of inhaled and intranasal niclosamide: a broad spectrum antiviral candidate for treatment of COVID-19",
"author": "Backer",
"journal-title": "Lancet Reg Health Eur",
"key": "10.1016/j.jiph.2024.03.030_bib48",
"volume": "4",
"year": "2021"
},
{
"DOI": "10.1371/journal.pone.0246803",
"article-title": "Development and evaluation of inhalable composite niclosamide-lysozyme particles: a broad-spectrum, patient-adaptable treatment for coronavirus infections and sequalae",
"author": "Brunaugh",
"doi-asserted-by": "crossref",
"issue": "2",
"journal-title": "PLoS One",
"key": "10.1016/j.jiph.2024.03.030_bib49",
"volume": "16",
"year": "2021"
},
{
"DOI": "10.1371/journal.pone.0198389",
"article-title": "A phase I study of niclosamide in combination with enzalutamide in men with castration-resistant prostate cancer",
"author": "Schweizer",
"doi-asserted-by": "crossref",
"issue": "6",
"journal-title": "PLoS One",
"key": "10.1016/j.jiph.2024.03.030_bib50",
"volume": "13",
"year": "2018"
},
{
"DOI": "10.1371/journal.pone.0202709",
"article-title": "Correction: a phase I study of niclosamide in combination with enzalutamide in men with castration-resistant prostate cancer",
"author": "Schweizer",
"doi-asserted-by": "crossref",
"issue": "8",
"journal-title": "PLoS One",
"key": "10.1016/j.jiph.2024.03.030_bib51",
"volume": "13",
"year": "2018"
},
{
"DOI": "10.1007/s11033-021-06770-7",
"article-title": "Niclosamide for Covid-19: bridging the gap",
"author": "Al-Kuraishy",
"doi-asserted-by": "crossref",
"first-page": "8195",
"issue": "12",
"journal-title": "Mol Biol Rep",
"key": "10.1016/j.jiph.2024.03.030_bib52",
"volume": "48",
"year": "2021"
}
],
"reference-count": 52,
"references-count": 52,
"relation": {},
"resource": {
"primary": {
"URL": "https://linkinghub.elsevier.com/retrieve/pii/S1876034124001060"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Infectious Diseases",
"Public Health, Environmental and Occupational Health",
"General Medicine"
],
"subtitle": [],
"title": "A randomized trial to assess the acceleration of viral clearance by the combination Favipiravir/Ivermectin/Niclosamide in mild-to-moderate COVID-19 adult patients (FINCOV)",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1016/elsevier_cm_policy",
"volume": "17"
}
siripongboonsitti6