Andrographolide was adopted
in 1 country.
Feb 19 |
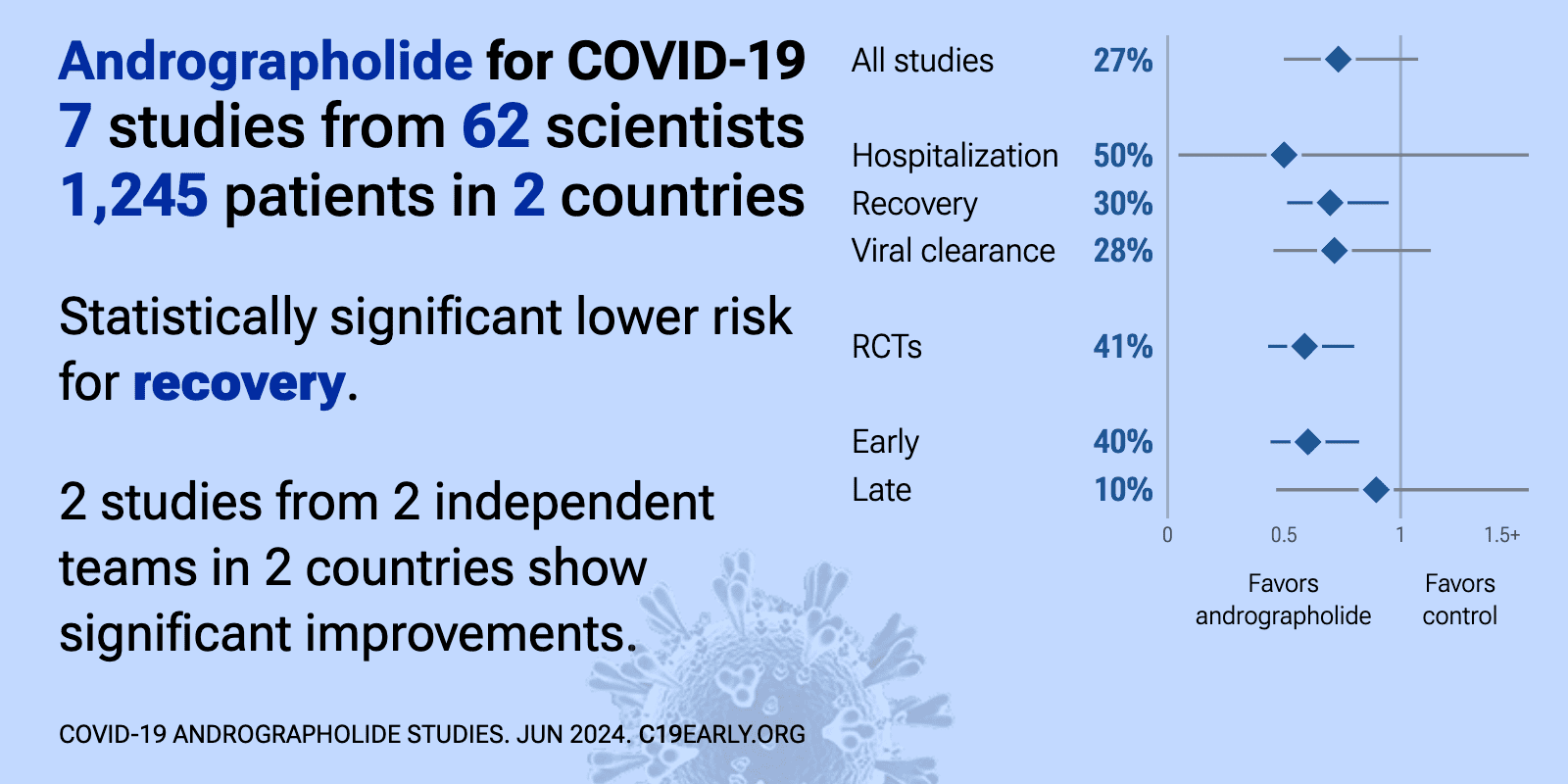
Andrographolide for COVID-19: real-time meta-analysis of 7 studies (Version 8) | |
| Significantly lower risk is seen for recovery. 2 studies from 2 independent teams in 2 countries show significant benefit. Meta-analysis using the most serious outcome reported shows 27% [-8‑50%] lower risk, without reaching st.. | ||
Jul 31 2025 |
et al., Phytomedicine Plus, doi:10.1016/j.phyplu.2025.100858 | Andrographis paniculata or Favipiravir in Mild COVID-19: A Randomized Control Trial |
| RCT 184 mild COVID-19 patients in Thailand showing no difference in pneumonia incidence or viral clearance between Andrographis paniculata and favipiravir. | ||
Jul 26 2025 |
et al., Phytomedicine Plus, doi:10.1016/j.phyplu.2025.100850 | Potential of diterpenoid andrographolide in COVID-19 therapy: an insight on its antiviral-, immunomodulatory-, anti-inflammatory-, antioxidant- and antithrombotic- properties |
| Review of andrographolide's potential for COVID-19, examining its antiviral, immunomodulatory, anti-inflammatory, antioxidant, and antithrombotic properties. Authors describe how andrographolide, a diterpenoid from Andrographis paniculata.. | ||
Jan 6 2025 |
et al., Pharmaceutical Biology, doi:10.1080/13880209.2024.2444446 | Non-linear oral bioavailability and clinical pharmacokinetics of high-dose Andrographis paniculata ethanolic extract: relevant dosage implications for COVID-19 treatment |
| Analysis of the pharmacokinetics and safety of high-dose Andrographis paniculata ethanolic extract. Authors observed non-linear oral bioavailability, with low plasma concentrations of key bioactive diterpenoids following ethanolic extract.. | ||
Nov 30 2024 |
et al., Journal of Traditional and Complementary Medicine, doi:10.1016/j.jtcme.2024.05.004 | In vivo evaluation of Andrographis paniculata and Boesenbergia rotunda extract activity against SARS-CoV-2 Delta variant in Golden Syrian hamsters: Potential herbal alternative for COVID-19 treatment |
| Syrian hamster study showing that Andrographis paniculata extract improves survival without reducing viral load in SARS-CoV-2 Delta variant infection, likely through anti-inflammatory effects. Treatment with A. paniculata extract led to 1.. | ||
Nov 25 2024 |
, K., Asian Pacific Journal of Tropical Biomedicine, doi:10.4103/apjtb.apjtb_751_23 | Antiviral efficacy of Andrographis paniculata and andrographolides: A narrative review |
| Review of the efficacy of Andrographis paniculata and andrographolides against viruses including SARS-CoV-2. Author discusses extracts and andrographolide derivatives that have shown immunomodulatory and antiviral effects in vitro and in .. | ||
Oct 7 2024 |
et al., Natural Product Communications, doi:10.1177/1934578X241288428 | Effects and Mechanisms of Andrographolide for COVID-19: A Network Pharmacology-Based and Experimentally Validated Study |
| In silico and in vitro study showing that andrographolide inhibits SARS-CoV-2 pseudovirus entry and replication and suppresses proinflammatory cytokine expression in BEAS-2B bronchial epithelial cells. Network pharmacology analysis identi.. | ||
Jul 1 2024 |
et al., Phytomedicine, doi:10.1016/j.phymed.2024.156279 (date from preprint) | Andrographolide attenuates SARS-CoV-2 infection via an up-regulation of glutamate-cysteine ligase catalytic subunit (GCLC) |
| In vitro study showing andrographolide attenuates infection of SARS-CoV-2 wildtype and omicron variants in human lung epithelial cells and monkey kidney cells. Proteomic analysis revealed andrographolide induces expression of the glutathi.. | ||
Jun 14 2024 |
et al., Antiviral Therapy, doi:10.1177/13596535241259952 | Andrographolide suppresses SARS-CoV-2 infection by downregulating ACE2 expression: A mechanistic study |
| In vitro study showing that andrographolide downregulates ACE2 expression and inhibits SARS-CoV-2 pseudovirus infection in a dose-dependent manner in human cells. Authors constructed a screening platform with a dual-luciferase reporter ve.. | ||
Apr 29 2024 |
et al., Scientific Reports, doi:10.1038/s41598-024-58532-7 | Cheminformatics approach to identify andrographolide derivatives as dual inhibitors of methyltransferases (nsp14 and nsp16) of SARS-CoV-2 |
| In silico study showing that andrographolide derivatives (PubChem CID 2734589 and 138968421) are potential dual inhibitors of SARS-CoV-2 methyltransferases nsp14 and nsp16, which are crucial for viral replication and evading host immune r.. | ||
Feb 22 2024 |
et al., bioRxiv, doi:10.1101/2024.02.21.581396 | The wide spectrum anti-inflammatory activity of andrographolide in comparison to NSAIDs: a promising therapeutic compound against the cytokine storm |
| In vitro study showing that andrographolide exhibits broad anti-inflammatory and cytokine inhibiting activity against lipopolysaccharide (LPS) and interferon-γ induced inflammation in murine RAW264.7 and human THP-1 macrophage cell lines... | ||
Feb 21 2024 |
et al., Scientific Reports, doi:10.1038/s41598-024-54722-5 | Synergistic inhibition effects of andrographolide and baicalin on coronavirus mechanisms by downregulation of ACE2 protein level |
| In vitro and mouse study showing that andrographolide and baicalin have synergistic antiviral effects against SARS-CoV-2. Andrographolide and baicalin inhibited binding between the SARS-CoV-2 spike protein and ACE2 receptor in human cells.. | ||
Feb 2 2024 |
et al., OBM Integrative and Complementary Medicine, doi:10.21926/obm.icm.2401013 | Efficacy and Safety of Andrographolide and Favipiravir Versus Favipiravir Monotherapy in Patients with Mild COVID-19 Infection: A Multicenter Randomized Controlled Trial |
| 37% improved recovery (p=0.005). Randomized controlled trial of 82 mild COVID-19 outpatients showing significantly greater reduction in cough and lower inflammatory markers at day 7. Symptomatic improvement was significant at day 7 when combining all symptoms reported, b.. | ||
Dec 29 2023 |
et al., Journal of Thai Traditional & Alternative Medicine, 21:3 | Randomized-Controlled Trial to Compare the Efficacy of Andrographis paniculata Powder and Favipiravir for the Treatment of Mild COVID-19 |
| RCT 231 mild COVID-19 outpatients in Thailand comparing Andrographis paniculata (AP) extract in two forms versus favipiravir, showing no significant differences between groups. Patients were randomized to either aerial parts AP extract (n.. | ||
Dec 21 2023 |
et al., 2023 IEEE 5th Eurasia Conference on Biomedical Engineering, Healthcare and Sustainability, doi:10.3390/engproc2023055081 | Dynamic Model of Andrographolide Therapy for COVID-19 |
| Pharmacokinetic and pharmacodynamic modeling of andrographolide for COVID-19. The model suggests that a dose of 60mg per day of andrographolide is optimal for reducing viral load and infection. The model incorporates the diffusion of the .. | ||
Dec 7 2023 |
et al., Journal of Ethnopharmacology, doi:10.1016/j.jep.2023.117535 | Efficacy and safety of Link Natural Sudarshana, an Ayurvedic herbal preparation in COVID-19 patients: A phase II multicenter double-blind randomized placebo-controlled trial |
| RCT 171 hospitalized COVID-19 patients in Sri Lanka, showing improved viral clearance and recovery, without statistical significance, for treatment with Link Natural Sudarshana (LNS). LNS is an Ayurvedic herbal preparation containing 49 m.. | ||
Nov 23 2023 |
et al., Research in Pharmaceutical Sciences, doi:10.4103/1735-5362.389947 | Andrographis paniculata extract versus placebo in the treatment of COVID-19: a double-blinded randomized control trial |
| 51% lower progression (p=0.25) and 8% improved recovery (p=0.33). RCT 165 low-risk mild COVID-19 patients in Thailand receiving either 180mg/day of Andrographis paniculata extract or placebo for 5 days. No significant difference was found between groups for disease progression, though A. paniculata show.. | ||
Nov 15 2023 |
et al., Journal of Ayurveda and Integrative Medicine, doi:10.1016/j.jaim.2023.100778 | Effectiveness of ayurvedic formulation, NAOQ19 along with standard care in the treatment of mild-moderate COVID-19 patients: A double blind, randomized, placebo-controlled, multicentric trial |
| 13% shorter hospitalization (p=0.05) and 12% faster viral clearance (p=0.02). RCT 150 mild-moderate COVID-19 patients showing faster viral clearance and shorter hospitalization with NAOQ19, a polyherbal formulation containing 13 antiviral/antinflammatory compounds. NAOQ19 ingredients include curcumin, andrographis,.. | ||
Nov 13 2023 |
et al., Scientific Reports, doi:10.1038/s41598-023-46249-y | Inhibitory efficiency of Andrographis paniculata extract on viral multiplication and nitric oxide production |
| In vitro study analyzing the antiviral and anti-inflammatory properties of extracts and fractions from Andrographis paniculata, against herpes simplex virus-1 (HSV-1) and influenza A virus H3N2 as surrogates for COVID-19. The results show.. | ||
Nov 9 2023 |
et al., GSC Biological and Pharmaceutical Sciences, doi:10.30574/gscbps.2023.25.2.0292 | Computational exploration of Andrographis paniculata herb compounds as potential antiviral agents targeting NSP3 (6W02) and NSP5 (7AR6) of SARS-COV-2 |
| In silico screening of 41 phytochemicals from Andrographis paniculata suggests 5 compounds - 12S-Hydroxyandrographolide, 14-Deoxy-11,12-didehydroandrographolide, Andrographolide, Andropanolide, and Isoandrographolide - have potential as a.. | ||
Sep 16 2023 |
et al., Natural Product Communications, doi:10.1177/1934578X231188861 | In Vitro Anti-SARS-CoV-2 Activities of Curcumin and Selected Phenolic Compounds |
| In vitro study showing that of 9 phenolic compounds tested, only curcumin inhibited SARS-CoV-2 cytopathic effects in infected monkey kidney Vero E6 cells. Curcumin showed antiviral activity against wildtype, alpha, delta, and omicron vari.. | ||
Aug 22 2023 |
et al., Pharmaceuticals, doi:10.3390/ph16091196 | Efficacy of Kan Jang® in Patients with Mild COVID-19: A Randomized, Quadruple-Blind, Placebo-Controlled Trial |
| 71% lower progression (p=0.03), 70% improved recovery (p=0.07), and 41% faster viral clearance (p=0.12). RCT 140 mild COVID-19 patients treated within 3 days of onset, showing lower progression and improved recovery with Kan Jang (andrographis + eleuthero). | ||
Aug 12 2023 |
et al., Phytomedicine, doi:10.1016/j.phymed.2023.155018 | Efficacy of Andrographis paniculata extract treatment in mild to moderate COVID-19 patients being treated with favipiravir: A double-blind, randomized, placebo-controlled study (APFaVi trial) |
| 86% lower need for oxygen therapy (p=0.24), 37% improved recovery (p=0.28), and 5% worse viral clearance (p=0.46). RCT 146 mild/moderate COVID-19 patients in Thailand, showing no significant difference in clinical outcomes. There were very few serious outcomes. | ||
Jun 30 2023 |
et al., Phytomedicine, doi:10.1016/j.phymed.2023.154753 | The effects and mechanisms of the anti-COVID-19 traditional Chinese medicine, Dehydroandrographolide from Andrographis paniculata (Burm.f.) Wall, on acute lung injury by the inhibition of NLRP3-mediated pyroptosis |
| In vitro and mouse study investigating the potential therapeutic effects of dehydroandrographolide (Deh), a compound from the herb Andrographis paniculata, in models of acute lung injury caused by COVID-19 infection. Deh reduced inflammat.. | ||
May 31 2023 |
et al., Archives of Internal Medicine Research, doi:10.26502/aimr.0146 | Effect of Andrographis paniculata Treatment for Nonimmune Patients with Early-Stage COVID-19 on the Prevention of Pneumonia: A Retrospective Cohort Study |
| 98% lower progression (p<0.0001). Retrospective 528 asymptomatic/mild patients in Thailand, showing lower progression to pneumonia with andrographis treatment. | ||
Feb 28 2023 |
et al., Phytomedicine Plus, doi:10.1016/j.phyplu.2022.100398 | A randomized controlled pilot study of add-on therapy of CIM-MEG19 (standardized Andrographis paniculata formulation) in mild to moderate COVID-19 |
| 91% lower progression (p=0.05), 33% faster recovery (p<0.0001), and 6% improved viral clearance (p=1). 80 mild/moderate hospitalized COVID-19 patients in India, 40 treated with CIM-MEG19 (standardized Andrographis paniculata formulation), showing faster recovery, lower progression, and favorable changes in inflammatory markers, however the.. | ||
Feb 21 2023 |
et al., AYU (An International Quarterly Journal of Research in Ayurveda), doi:10.4103/ayu.ayu_92_22 | Safety and efficacy of COROPROTECT kit as an add-on therapy in the management of mild-to-moderate COVID-19: A randomized, placebo-controlled trial |
| 86% improved recovery (p<0.0001) and 53% improved viral clearance (p<0.0001). RCT with 300 mild to moderate hospitalized COVID-19 patients, showing faster recovery, faster viral clearance, and a reduction in inflammatory markers with COROPROTECT, which includes curcumin, andrographis, and several additional treatme.. | ||
Feb 10 2023 |
et al., Journal of Biomolecular Structure and Dynamics, doi:10.1080/07391102.2023.2174596 | Investigating the binding affinity of andrographolide against human SARS-CoV-2 spike receptor-binding domain through docking and molecular dynamics simulations |
| In silico study showing that andrographolide binds strongly to the SARS-CoV-2 spike protein receptor-binding domain (RBD). Authors used molecular docking and dynamics simulations to demonstrate that andrographolide forms stable non-covale.. | ||
Jan 12 2023 |
et al., Bioinformatics and Biology Insights, doi:10.1177/11779322221149622 | The Potential of Ameliorating COVID-19 and Sequelae From Andrographis paniculata via Bioinformatics |
| In silico study identifying multiple compounds including andrographolide, quercetin, and hydroxychloroquine (used as a reference) as promising inhibitors of SARS-CoV-2. Authors note the potential synergistic effect of multiple compounds. | ||
Dec 31 2022 |
et al., OSIR, 15:4 | SARS-CoV-2 Clearance from Andrographis paniculata, Boesenbergia rotunda, and Favipiravir among Mild COVID-19 Cases in Klong Prem Central Prison during Mid-2021: a Retrospective Study |
| 19% improved viral clearance (p=0.61). Retrospective 120 patients in Thailand, showing improved viral clearance with andrographis compared with favipiravir. | ||
Sep 22 2022 |
et al., International Journal of Molecular Sciences, doi:10.3390/ijms231911131 | Molecular Docking and In-Silico Analysis of Natural Biomolecules against Dengue, Ebola, Zika, SARS-CoV-2 Variants of Concern and Monkeypox Virus |
| In silico study showing that the phytochemical andrographolide derived from Andrographis paniculata binds strongly to the spike proteins of six SARS-CoV-2 variants, including the B.1.1.7 UK, B.1.351 South African, P.1 Japan/Brazil, B.1.42.. | ||
Aug 10 2022 |
et al., Frontiers in Medicine, doi:10.3389/fmed.2022.947373 | Use of Andrographis paniculata (Burm.f.) Wall. ex Nees and risk of pneumonia in hospitalised patients with mild coronavirus disease 2019: A retrospective cohort study |
| 26% higher progression (p=0.4). Retrospective 605 hospitalized patients in Thailand, showing higher progression with andrographis, without statistical significance. | ||
Jul 13 2022 |
et al., Molecules, doi:10.3390/molecules27144479 | A Comprehensive Review of Andrographis paniculata (Burm. f.) Nees and Its Constituents as Potential Lead Compounds for COVID-19 Drug Discovery |
| Review of Andrographis paniculata extracts and compounds like andrographolide with antiviral, anti-inflammatory, and immunomodulatory properties that could help treat COVID-19 symptoms and progression. In silico studies suggest these comp.. | ||
Jun 30 2022 |
et al., Proceedings Universitas Muhammadiyah Yogyakarta Undergraduate Conference, doi:10.18196/umygrace.v2i2.418 | Potency Of Andrographolide, L-Mimosine And Asiaticoside Compound As Antiviral For Covid-19 Based On In Silico Method |
| In silico study showing that andrographolide, L-mimosine, and asiaticoside compounds from Andrographis paniculata, Mimosa pudica and Centella asiatica plants have potential as COVID-19 antivirals by binding to SARS-CoV-2 3CLpro, NSP3, and.. | ||
May 30 2022 |
et al., NCT05178173 | Andrographis Paniculata vs Boesenbergia Rotunda vs Control in Asymptomatic COVID-19 |
| Estimated 3,060 patient andrographolide early treatment RCT with results not reported over 3 years after estimated completion. | ||
Mar 28 2022 |
et al., Biointerface Research in Applied Chemistry, doi:10.33263/BRIAC132.155 | Identification of Potential Semisynthetic Andrographolide Derivatives to Combat COVID-19 by Targeting the SARS-COV-2 Spike Protein and Human ACE2 Receptor– An In-silico Approach |
| In silico study showing that semisynthetic andrographolide derivatives have potential to inhibit SARS-CoV-2 infection by targeting the spike protein and human ACE2 receptor. Docking studies found that derivatives AGP15 and AGP14 had the h.. | ||
Jan 31 2022 |
et al., The American Journal of Chinese Medicine, doi:10.1142/S0192415X22500732 | In Silico Prediction of Andrographolide Dosage Regimens for COVID-19 Treatment |
| In silico study showing potential benefits of andrographolide for COVID-19 treatment and optimal dosage regimens predicted by physiologically-based pharmacokinetic and pharmacodynamic (PBPK/PD) modeling. Authors find that oral crude extra.. | ||
Dec 31 2021 |
et al., RSC Advances, doi:10.1039/D0RA10529E | Combination of system biology to probe the anti-viral activity of andrographolide and its derivative against COVID-19 |
| In silico study showing that andrographolide and 14-deoxy-11,12-didehydroandrographolide from Andrographis paniculata modulate immune pathways and bind to SARS-CoV-2 papain-like protease, main protease, and spike protein. Authors find tha.. | ||
Dec 27 2021 |
et al., Research Square, doi:10.21203/rs.3.rs-1165680/v1 | An integrative approach to clinical recovery for COVID-19 patients using an Ayurvedic formulation: A multicentric double-blind randomized control trial |
| 89% improved recovery (p=0.05) and 24% improved viral clearance (p=0.47). Small RCT with 39 patients treated with NOQ19 and 37 placebo patients, showing improved recovery, without statistical significance. NOQ19 has multiple ingredients including curcumin, andrographis, and antiandrogen glycyrrhiza glabra. | ||
Oct 11 2021 |
et al., Evidence-Based Complementary and Alternative Medicine, doi:10.1155/2021/8447545 | A Randomized, Double-Blind, Placebo-Controlled Study to Assess the Efficacy and Safety of a Nutritional Supplement (ImmuActive) for COVID-19 Patients |
| 43% improved recovery (p=0.004) and 6% faster viral clearance (p=0.47). RCT 100 patients in India, 50 treated with ImmuActive (curcumin, andrographolides, resveratrol, zinc, selenium, and piperine), showing improved recovery with treatment. | ||
Jul 11 2021 |
et al., Archives of Internal Medicine Research, doi:10.26502/aimr.0125 (date from preprint) | Efficacy and Safety of Andrographis Paniculata Extract in Patients with Mild COVID-19: A Randomized Controlled Trial |
| 86% lower progression (p=0.11) and 40% improved viral clearance (p=0.11). RCT 63 mild COVID-19 patients showing lower progression and improved viral clearance with andrographis, without statistical significance. | ||
Jun 24 2021 |
et al., Frontiers in Immunology, doi:10.3389/fimmu.2021.648250 | A Computational Approach Identified Andrographolide as a Potential Drug for Suppressing COVID-19-Induced Cytokine Storm |
| In silico study showing that andrographolide, a compound from Andrographis paniculata, may reduce cytokine storm in COVID-19 by targeting the TNF signaling pathway. Authors found that andrographolide binds to TNF and NFkB1 proteins, poten.. | ||
May 12 2021 |
et al., Phytotherapy Research, doi:10.1002/ptr.7141 | Efficacy and safety of Xiyanping injection in the treatment of COVID‐19: A multicenter, prospective, open‐label and randomized controlled trial |
| 92% lower severe cases (p=0.03), 48% improved recovery (p=0.008), and 53% improved viral clearance (p=0.0001). RCT 130 hospitalized COVID-19 patients in China, showing lower progression and improved recovery with Xiyanping injection (9-dehydro-17-hydro-andrographolide and sodium 9-dehydro-17-hydro-andrographolide-19-yl sulfate, which are derived f.. | ||
Oct 16 2020 |
et al., Future Journal of Pharmaceutical Sciences, doi:10.1186/s43094-020-00126-x | Activity of phytochemical constituents of Curcuma longa (turmeric) and Andrographis paniculata against coronavirus (COVID-19): an in silico approach |
| In silico study of several phytochemical compounds from Curcuma longa (turmeric) and Andrographis paniculata for their potential activity against COVID-19 by targeting the SARS-CoV-2 main protease. Molecular docking analysis found the tur.. | ||
Jun 18 2020 |
et al., Research Square, doi:10.21203/rs.3.rs-35800/v1 | The role of andrographolide and its derivative in COVID-19 associated proteins and immune system |
| In silico study showing that andrographolide and its derivative 14-deoxy-11,12-didehydroandrographolide from Andrographis paniculata may be beneficial for COVID-19 by binding to key SARS-CoV-2 proteins and modulating immune pathways. Auth.. | ||