Efficacy and Safety of Andrographis Paniculata Extract in Patients with Mild COVID-19: A Randomized Controlled Trial
et al., Archives of Internal Medicine Research, doi:10.26502/aimr.0125, TCTR20210708001, Jul 2021 (preprint)
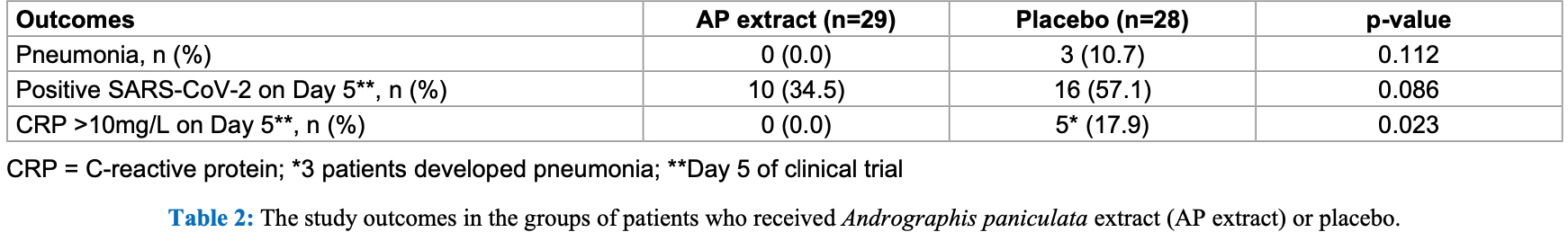
RCT 63 mild COVID-19 patients showing lower progression and improved viral clearance with andrographis, without statistical significance.
|
risk of progression, 85.9% lower, RR 0.14, p = 0.11, treatment 0 of 29 (0.0%), control 3 of 28 (10.7%), NNT 9.3, relative risk is not 0 because of continuity correction due to zero events (with reciprocal of the contrasting arm).
|
|
risk of no viral clearance, 39.7% lower, RR 0.60, p = 0.11, treatment 10 of 29 (34.5%), control 16 of 28 (57.1%), NNT 4.4, day 5.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Wanaratna et al., 11 Jul 2021, Double Blind Randomized Controlled Trial, placebo-controlled, Thailand, peer-reviewed, 7 authors, study period December 2020 - March 2021, trial TCTR20210708001.
Efficacy and Safety of Andrographis Paniculata Extract in Patients with Mild COVID-19: A Randomized Controlled Trial
Archives of Internal Medicine Research, doi:10.26502/aimr.0125
Objectives: To assess the efficacy and safety of Andrographis paniculata extract (APE) in adults with mild COVID-19. Methods: Sixty-three adults aged 18-60 years, without co-morbidity, with laboratory-confirmed mild COVID-19, were randomized 1:1 to receive APE (60 mg andrographolide, t.i.d, for 5 days) or placebo within 24 hours after admission, plus standard supportive care. The outcomes were clinical recovery rates by Day 5 using self-assessment scores, pneumonia by chest X-rays, nasopharyngeal SARS-CoV-2 detection by rRT-PCR on Day 5, changes of serum CRP levels, and adverse drug reactions. Chest X-rays and blood tests for CRP, liver and renal function, were performed on Days 1, 3, and 5. Results: Baseline characteristics of patients in the APE-treatment (n=29) and placebo-control (n=28) groups were comparable. None had self-assessment scores showing complete clinical recovery by Day 5. Pneumonia occurred in 0/29 (0%) versus 3/28 (10.7%), (p=0.112). On Day 5, patients with SARS-CoV-2 detection were 10/29 (34.5%) versus 16/28 (57.1%), (p=0.086); patients with CRP >10 mg/L were 0/29 (0%) versus 5/28 (17.9%), (p=0.023), for APE-treatment and placebo-control groups, respectively. All three patients with pneumonia had substantially rising serum CRP; and high CRP levels on Day 5. None had evidence of hematologic, hepatic or renal impairment.
Conclusion: Even though the study was limited by small sample size, our findings suggested promising efficacy and safety of the APE-treatment regimen in adults with mild COVID-19. Further studies, with adequate power to assure these findings, are required.
Potential Conflicts of Interest The authors have no conflicts of interest to declare.
Ethics Approval and Consent to Participate Ethical approval was obtained from the Ethics Committee for Research in Human Subjects in the Fields of Thai Traditional and Alternative Medicine (No.12-2563). All participants gave written informed consent.
References
Banerjee, Kar, Mukherjee, Immunoprotective potential of Ayurvedic herb Kalmegh (Andrographis paniculata) against respiratory viral infections -LC-MS/ MS and network pharmacology analysis, Phytochem Anal
Dai, Chen, Chai, Overview of pharmacological activities of Andrographis paniculata and its major compound andrographolide, Crit Rev Food Sci Nutr
Enmozhi, Raja, Sebastine, Andrographolide as a potential inhibitor of SARS-CoV-2 main protease: an in silico approach, J Biomol Struct Dyn
Hiremath, Kumar, Nandan, In silico docking analysis revealed the potential of phytochemicals present in Phyllanthus amarus and Andrographis paniculata, used in Ayurveda medicine in inhibiting SARS-CoV-2, Biotech
Hossain, Urbi, Karuniawati, Andrographis paniculata (Burm. f.) Wall. ex Nees: An updated review of phytochemistry, antimicrobial pharmacology, and clinical safety and efficacy, Life
Mueller, Tamura, Crowley, Inflammatory biomarker trends predict respiratory decline in COVID-19 patients, Cell Rep Med
Murugan, Pandian, Jeyakanthan, Computational investigation on Andrographis paniculata phytochemicals to evaluate their potency against SARS-CoV-2 in comparison to known antiviral compounds in drug trials, J Biomol Struct Dyn
Phumiamorn, Sapsutthipas, Pruksakorn, In vitro study on antiviral activity of Andrographis paniculata against COVID-19
Potempa, Rajab, Hart, Insights into the Use of C-Reactive Protein as a Diagnostic Index of Disease Severity in COVID-19 Infections, Am J Trop Med Hyg
Sa-Ngiamsuntorn, Suksatu, Pewkliang, Anti-SARS-CoV-2 Activity of Andrographis paniculata extract and its major component andrographolide in human lung epithelial cells and cytotoxicity evaluation in major organ cell representatives, J Nat Prod
Shi, Huang, Chen, Andrographolide and its fluorescent derivative inhibit the main proteases of 2019-nCoV and SARS-CoV through covalent linkage, Biochem Biophys Res Commun
Stringer, Braude, Myint, COPE Study Collaborators. The role of C-reactive protein as a prognostic marker in COVID-19, Int J Epidemiol
Suriyo, Pholphana, Ungtrakul, Clinical parameters following multiple oral dose administration of a standardized Andrographis paniculata capsule in healthy Thai subjects, Planta Med
Thamlikitkul, Dechatiwongse, Theerapong, Efficacy of Andrographis paniculata, Nees for pharyngotonsillitis in adults, J Med Assoc Thai
Tsang, Colley, Lynd, Inadequate statistical power to detect clinically significant differences in adverse event rates in randomized controlled trials, J Clin Epidemio
Van Eijk, Binkhorst, Bourgonje, COVID-19: immunopathology, pathophysiological mechanisms, and treatment options, J Pathol
Wang, C-reactive protein levels in the early stage of COVID-19, Med Mal Infect
Worakunphanich, Thavorncharoensap, Youngkong, Safety of Andrographis paniculata: A systematic review and meta-analysis, Pharmacoepidemiol Drug Saf
Wu, Mcgoogan, Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention, JAMA
Zhang, Lv, Zhou, Efficacy and safety of Xiyanping injection in the treatment of COVID-19: A multicenter, prospective, open-label and randomized controlled trial, Phytother Res
DOI record:
{
"DOI": "10.26502/aimr.0125",
"ISSN": [
"2688-5654"
],
"URL": "http://dx.doi.org/10.26502/aimr.0125",
"author": [
{
"affiliation": [],
"family": "Wanaratna",
"given": "Kulthanit",
"sequence": "first"
},
{
"affiliation": [],
"family": "Leethong",
"given": "Pornvimol",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Inchai",
"given": "Nitapha",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Chueawiang",
"given": "Wararath",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Sriraksa",
"given": "Pantitra",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Tabmee",
"given": "Anutida",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Sirinavin",
"given": "Sayomporn",
"sequence": "additional"
}
],
"container-title": "Archives of Internal Medicine Research",
"container-title-short": "Arch Intern Med Res",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2022,
8,
21
]
],
"date-time": "2022-08-21T14:52:19Z",
"timestamp": 1661093539000
},
"deposited": {
"date-parts": [
[
2022,
8,
21
]
],
"date-time": "2022-08-21T14:52:33Z",
"timestamp": 1661093553000
},
"indexed": {
"date-parts": [
[
2022,
10,
26
]
],
"date-time": "2022-10-26T04:33:21Z",
"timestamp": 1666758801781
},
"is-referenced-by-count": 1,
"issue": "03",
"issued": {
"date-parts": [
[
2022
]
]
},
"journal-issue": {
"issue": "03",
"published-online": {
"date-parts": [
[
2022
]
]
},
"published-print": {
"date-parts": [
[
2022
]
]
}
},
"member": "11040",
"original-title": [],
"prefix": "10.26502",
"published": {
"date-parts": [
[
2022
]
]
},
"published-online": {
"date-parts": [
[
2022
]
]
},
"published-print": {
"date-parts": [
[
2022
]
]
},
"publisher": "Fortune Journals",
"reference-count": 0,
"references-count": 0,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.fortunejournals.com/articles/efficacy-and-safety-of-andrographis-paniculata-extract-in-patients-with-mild-covid19-a-randomized-controlled-trial.html"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"General Medicine"
],
"subtitle": [],
"title": "Efficacy and Safety of Andrographis Paniculata Extract in Patients with Mild COVID-19: A Randomized Controlled Trial",
"type": "journal-article",
"volume": "05"
}