Mar 1 |
Xiannuoxin for COVID-19: real-time meta-analysis of 2 studies | |
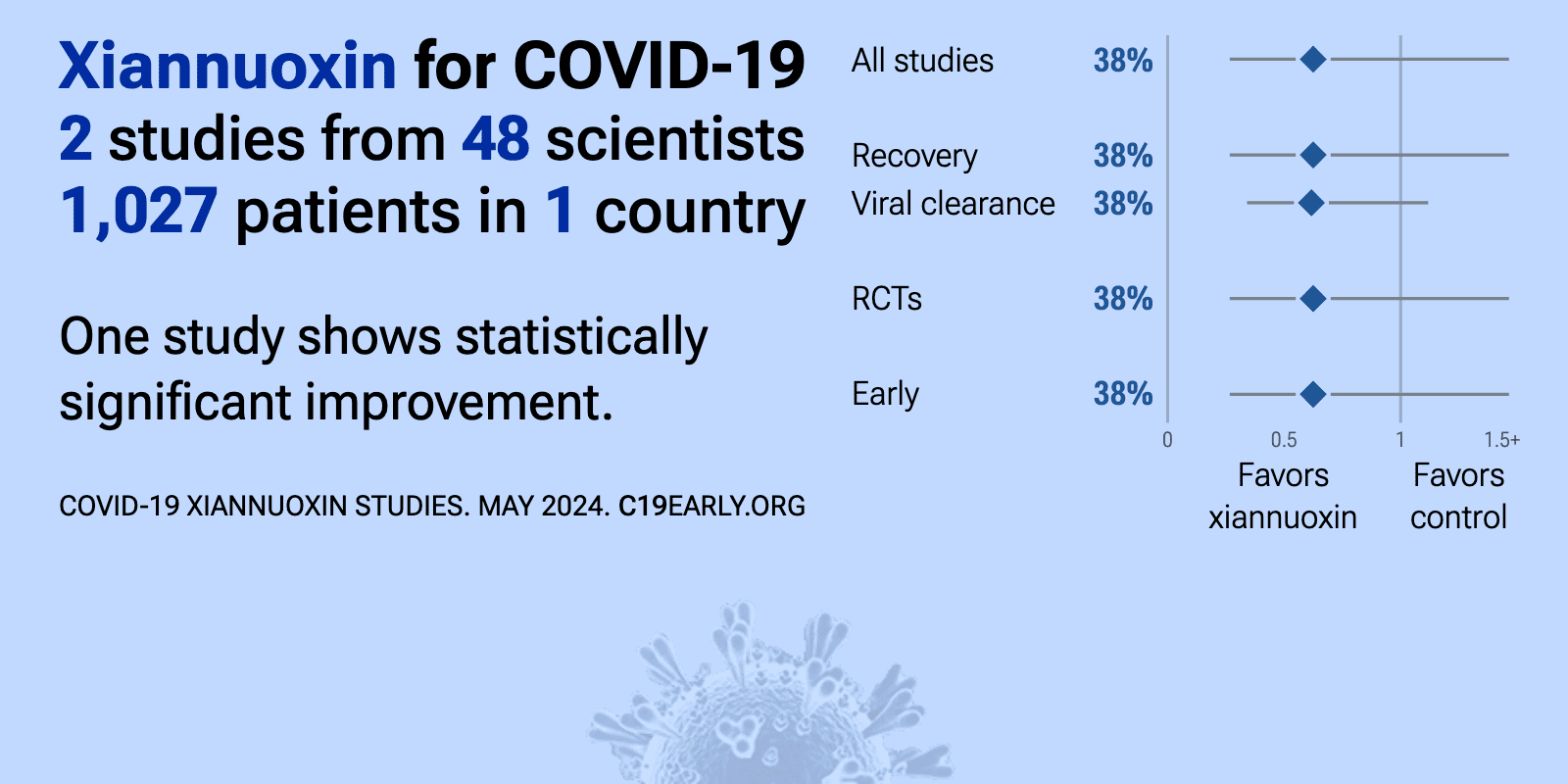
| Meta-analysis using the most serious outcome reported shows 38% [-46‑73%] lower risk, without reaching statistical significance. Currently all studies are RCTs. One study shows significant benefit. Studies to date are from only.. | ||
Jul 1 2025 |
et al., BMC Infectious Diseases, doi:10.1186/s12879-025-11195-9 | Real-world effectiveness of simnotrelvir-ritonavir versus nirmatrelvir-ritonavir in hospitalized patients with COVID-19 during the omicron wave in China: a retrospective cohort study |
| Retrospective 585 hospitalized COVID-19 patients in China showing that xiannuoxin provided better clinical improvement than paxlovid. There were no significant differences for progression, mortality, or respiratory support. | ||
Apr 2 2025 |
et al., Antimicrobial Agents and Chemotherapy, doi:10.1128/aac.01556-24 | Potent antiviral activity of simnotrelvir against key epidemic SARS-CoV-2 variants with a high resistance barrier |
| In vitro and clinical study showing potent antiviral activity of simnotrelvir against SARS-CoV-2 variants with a high resistance barrier. Authors demonstrated simnotrelvir's efficacy against multiple Omicron variants including BA.1, BA.4,.. | ||
Jan 15 2024 |
et al., Toxics, doi:10.3390/toxics12010073 | Ritonavir Has Reproductive Toxicity Depending on Disrupting PI3K/PDK1/AKT Signaling Pathway |
| In vitro study on boar spermatozoa showing that the HIV drug ritonavir (part of paxlovid and xiannuoxin) causes reproductive toxicity by disrupting the PI3K/PDK1/AKT signaling pathway. Ritonavir suppressed sperm functions including motili.. | ||
Dec 31 2023 |
et al., European Journal of Pharmaceutical Sciences, doi:10.1016/j.ejps.2023.106598 | A first-in-human phase 1 study of simnotrelvir, a 3CL-like protease inhibitor for treatment of COVID-19, in healthy adult subjects |
| RCT 72 healthy subjects evaluating safety, tolerability, and pharmacokinetics of simnotrelvir (SSD8432, SIM0417), a 3CL protease inhibitor, alone or co-administered with ritonavir at various single and multiple ascending oral doses and un.. | ||
Dec 31 2023 |
, E., Aging and disease, doi:10.14336/AD.2023.0318 | Mitigating COVID-19 Mortality and Morbidity in China's Aging Population: A Focus on Available Medications and Future Developments |
| Review focusing on 3CL protease inhibitors. First generation inhibitors like paxlovid and simnotrelvir require boosting with ritonavir, which can cause drug-drug interactions and other issues. Second generation inhibitors like ensitrelvir.. | ||
Oct 13 2023 |
et al., Nature Communications, doi:10.1038/s41467-023-42102-y | Structure-based development and preclinical evaluation of the SARS-CoV-2 3C-like protease inhibitor simnotrelvir |
| In vitro and mouse study showing the development and preclinical evaluation of the SARS-CoV-2 3C-like protease (3CLpro) inhibitor simnotrelvir (SSD8432, SIM0417, part of xiannuoxin) as an orally bioavailable COVID-19 therapeutic agent... | ||
Sep 30 2023 |
et al., The Lancet Regional Health - Western Pacific, doi:10.1016/j.lanwpc.2023.100835 | Efficacy and safety of SIM0417 (SSD8432) plus ritonavir for COVID-19 treatment: a randomised, double-blind, placebo-controlled, phase 1b trial |
| 68% faster recovery (p=0.06) and 38% improved viral clearance (p=0.11). RCT 32 adults with asymptomatic, mild or moderate COVID-19 in China showing greater viral load reduction and faster time to symptom alleviation with xuannuoxin (simnotrelvir plus ritonavir) compared to placebo. All adverse events were mild. | ||
Jul 31 2023 |
et al., Progress in Pharmaceutical Sciences, doi:10.20053/j.issn1001-5094.2023.07.002 | XIANNUOXIN®: China’s First Anti-SARS-CoV-2 Drug Targeting 3C-like Protease |
| Review of the development and approval of Xiannuoxin, the first domestic anti-COVID-19 drug targeting the 3C-like protease (3CLpro) approved in China. Xiannuoxin was jointly developed by Simcere Pharmaceutical Group, Shanghai Institute of.. | ||
Jan 29 2023 |
et al., New England Journal of Medicine, doi:10.1056/NEJMoa2301425 (press release 1/29/2023) | Oral Simnotrelvir for Adult Patients with Mild-to-Moderate Covid-19 |
| 17% faster recovery (p=0.003). RCT 1,208 outpatients with mild-moderate COVID-19 in China, showing faster symptom resolution (by 35.8 hours) and faster viral clearance with xiannuoxin (simnotrelvir plus ritonavir) twice daily for 5 days. | ||
References