Efficacy and safety of SIM0417 (SSD8432) plus ritonavir for COVID-19 treatment: a randomised, double-blind, placebo-controlled, phase 1b trial
et al., The Lancet Regional Health - Western Pacific, doi:10.1016/j.lanwpc.2023.100835, NCT05369676, Sep 2023
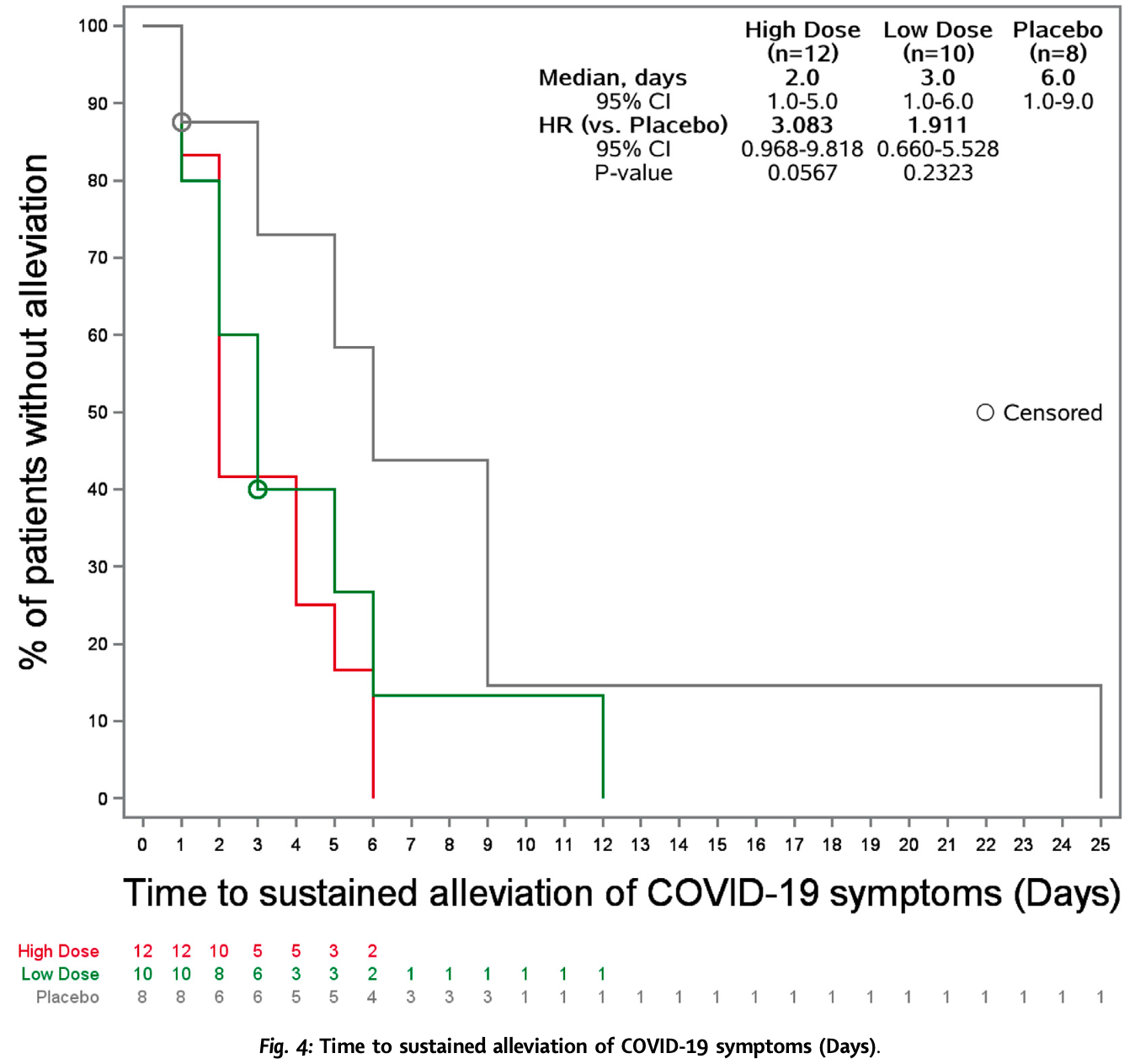
RCT 32 adults with asymptomatic, mild or moderate COVID-19 in China showing greater viral load reduction and faster time to symptom alleviation with xuannuoxin (simnotrelvir plus ritonavir) compared to placebo. All adverse events were mild.
Standard of Care (SOC) for COVID-19 in the study country,
China, is average with moderate efficacy for approved treatments1.
|
recovery time, 67.5% lower, HR 0.32, p = 0.06, treatment 12, control 8, inverted to make HR<1 favor treatment, high dose.
|
|
recovery time, 47.7% lower, HR 0.52, p = 0.23, treatment 10, control 8, inverted to make HR<1 favor treatment, low dose.
|
|
viral load, 38.3% lower, relative load 0.62, p = 0.11, treatment mean 2.21 (±1.21) n=12, control mean 3.58 (±2.44) n=8, high dose, mid-recovery, day 7.
|
|
viral load, 14.2% lower, relative load 0.86, p = 0.63, treatment mean 3.07 (±2.17) n=12, control mean 3.58 (±2.44) n=8, low dose, mid-recovery, day 7.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Wang et al., 30 Sep 2023, Double Blind Randomized Controlled Trial, placebo-controlled, China, peer-reviewed, median age 39.5, 17 authors, study period 12 May, 2022 - 29 August, 2022, average treatment delay 4.1 days, trial NCT05369676 (history).
Contact: luhongzhou@fudan.edu.cn, shenjingshan@simm.ac.cn, renhong.tang@simceregroup.com.
Efficacy and safety of SIM0417 (SSD8432) plus ritonavir for COVID-19 treatment: a randomised, double-blind, placebo-controlled, phase 1b trial
The Lancet Regional Health - Western Pacific, doi:10.1016/j.lanwpc.2023.100835
Background SIM0417 (SSD8432) is an orally administered coronavirus main proteinase (3CL pro ) inhibitor with potential anti-SARS-CoV-2 activity. This study aimed to evaluate the efficacy and safety of SIM0417 plus ritonavir (a pharmacokinetic enhancer) in adults with COVID-19. Methods This was a randomised, double-blind, placebo-controlled, phase 1b study in China. Adults with asymptomatic infection, mild or moderate COVID-19 were randomly assigned (3:3:2) to receive either 750 mg SIM0417 plus 100 mg ritonavir, 300 mg SIM0417 plus 100 mg ritonavir or placebo every 12 h for 10 doses. The main efficacy endpoints included SARS-CoV-2 viral load, proportion of participants with positive SARS-CoV-2 nucleic acid test and time to alleviation of COVID-19 symptoms. This trial is registered with ClinicalTrials.gov, NCT05369676. Findings Between May 12 and August 29, 2022, 32 participants were enrolled and randomised to high dose group (n = 12), low dose group (n = 12) or placebo (n = 8). The viral load change from baseline in high dose group was statistically lower compared with placebo, with a maximum mean difference of -2.16 ± 0.761 log 10 copies/mL (p = 0.0124) on Day 4. The proportion of positive SARS-CoV-2 in both active groups were lower than the placebo. The median time to sustained alleviation of COVID-19 symptoms was 2.0 days in high dose group versus 6.0 days in the placebo group (HR = 3.08, 95% CI 0.968-9.818). SIM0417 plus ritonavir were well tolerated with all adverse events in grade 1. Interpretation SIM0417 plus ritonavir was generally well tolerated. The efficacy of SIM0417 showed a monotonic dose-response relationship, and the 750 mg SIM0417 plus 100 mg ritonavir was selected as the recommended clinical dose.
Appendix A. Supplementary data Supplementary data related to this article can be found at https://doi. org/10.1016/j.lanwpc.2023.100835.
References
Alfano, Ferrari, Fontana, Hypokalemia in patients with COVID-19, Clin Exp Nephrol
Arribas, Bhagani, Lobo, Randomized trial of molnupiravir or placebo in patients hospitalized with covid-19, NEJM Evidence
Cameroni, Bowen, Rosen, Broadly neutralizing antibodies overcome SARS-CoV-2 Omicron antigenic shift, Nature
Chen, Li, Song, Hypokalemia and clinical implications in patients with coronavirus disease 2019 (COVID-19), medRxiv, doi:10.1101/2020.02.27.20028530
Chen, Xiao, Fang, Lv, Yao et al., Correlation analysis between the viral load and the progression of COVID-19, Comput Math Methods Med
Cohen, Wohl, Fischer, Ii, Smith et al., Outpatient treatment of severe acute respiratory syndrome coronavirus 2 infection to prevent coronavirus disease 2019 progression, Clin Infect Dis
Dadras, Afsahi, Pashaei, The relationship between COVID-19 viral load and disease severity: a systematic review, Immun Inflamm Dis
El-Shabasy, Nayel, Taher, Abdelmonem, Shoueir et al., Three waves changes, new variant strains, and vaccination effect against COVID-19 pandemic, Int J Biol Macromol
Fernandes, Inchakalody, Merhi, Emerging COVID-19 variants and their impact on SARS-CoV-2 diagnosis, therapeutics and vaccines, Ann Med
Forchette, Sebastian, Liu, A comprehensive review of COVID-19 virology, vaccines, variants, and therapeutics, Curr Med Sci
Gholamhoseini, Yazdi-Feyzabadi, Goudarzi, Mehrolhassani, Safety and efficacy of remdesivir for the treatment of COVID-19: a systematic review and meta-analysis, J Pharm Pharm Sci
Gupta, Gonzalez-Rojas, Juarez, Early treatment for covid-19 with SARS-CoV-2 neutralizing antibody sotrovimab, N Engl J Med
Hammond, Leister-Tebbe, Gardner, Oral nirmatrelvir for high-risk, nonhospitalized adults with covid-19, N Engl J Med
Hurt, Wheatley, Neutralizing antibody therapeutics for COVID-19, Viruses
Kumari, Lu, Li, A critical overview of current progress for COVID-19: development of vaccines, antiviral drugs, and therapeutic antibodies, J Biomed Sci
Liu, Liang, Xin, The development of Coronavirus 3C-Like protease (3CL(pro)) inhibitors from 2010 to 2020, Eur J Med Chem
Mandal, Wenban, Heer, Kho, Missouris, Covid-19, hypokalaemia and the renin-angiotensin-aldosterone system, Ann Med Surg (Lond)
Soufi, Iravani, Potential inhibitors of SARS-CoV-2: recent advances, J Drug Target
Tian, Liu, Liang, An update review of emerging smallmolecule therapeutic options for COVID-19, Biomed Pharmacother
Tsukagoshi, Shinoda, Saito, Relationships between viral load and the clinical course of COVID-19, Viruses
DOI record:
{
"DOI": "10.1016/j.lanwpc.2023.100835",
"ISSN": [
"2666-6065"
],
"URL": "http://dx.doi.org/10.1016/j.lanwpc.2023.100835",
"alternative-id": [
"S2666606523001530"
],
"article-number": "100835",
"assertion": [
{
"label": "This article is maintained by",
"name": "publisher",
"value": "Elsevier"
},
{
"label": "Article Title",
"name": "articletitle",
"value": "Efficacy and safety of SIM0417 (SSD8432) plus ritonavir for COVID-19 treatment: a randomised, double-blind, placebo-controlled, phase 1b trial"
},
{
"label": "Journal Title",
"name": "journaltitle",
"value": "The Lancet Regional Health - Western Pacific"
},
{
"label": "CrossRef DOI link to publisher maintained version",
"name": "articlelink",
"value": "https://doi.org/10.1016/j.lanwpc.2023.100835"
},
{
"label": "Content Type",
"name": "content_type",
"value": "article"
},
{
"label": "Copyright",
"name": "copyright",
"value": "© 2023 Published by Elsevier Ltd."
}
],
"author": [
{
"affiliation": [],
"family": "Wang",
"given": "Fuxiang",
"sequence": "first"
},
{
"affiliation": [],
"family": "Xiao",
"given": "Wen",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Tang",
"given": "Yimin",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Cao",
"given": "Mengli",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Shu",
"given": "Dan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Asakawa",
"given": "Tetsuya",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Xu",
"given": "Yechun",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Jiang",
"given": "Xiangrui",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Zhang",
"given": "Leike",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Wang",
"given": "Wei",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Tang",
"given": "Jianxing",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Huang",
"given": "Yuansheng",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Yang",
"given": "Yang",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Yang",
"given": "Yumei",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Tang",
"given": "Renhong",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Shen",
"given": "Jingshan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lu",
"given": "Hongzhou",
"sequence": "additional"
}
],
"container-title": "The Lancet Regional Health - Western Pacific",
"container-title-short": "The Lancet Regional Health - Western Pacific",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"thelancet.com",
"elsevier.com",
"sciencedirect.com"
]
},
"created": {
"date-parts": [
[
2023,
7,
11
]
],
"date-time": "2023-07-11T17:01:33Z",
"timestamp": 1689094893000
},
"deposited": {
"date-parts": [
[
2023,
11,
6
]
],
"date-time": "2023-11-06T01:56:06Z",
"timestamp": 1699235766000
},
"indexed": {
"date-parts": [
[
2024,
1,
25
]
],
"date-time": "2024-01-25T13:00:01Z",
"timestamp": 1706187601098
},
"is-referenced-by-count": 4,
"issued": {
"date-parts": [
[
2023,
9
]
]
},
"language": "en",
"license": [
{
"URL": "https://www.elsevier.com/tdm/userlicense/1.0/",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2023,
9,
1
]
],
"date-time": "2023-09-01T00:00:00Z",
"timestamp": 1693526400000
}
},
{
"URL": "http://creativecommons.org/licenses/by-nc-nd/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2023,
6,
19
]
],
"date-time": "2023-06-19T00:00:00Z",
"timestamp": 1687132800000
}
}
],
"link": [
{
"URL": "https://api.elsevier.com/content/article/PII:S2666606523001530?httpAccept=text/xml",
"content-type": "text/xml",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://api.elsevier.com/content/article/PII:S2666606523001530?httpAccept=text/plain",
"content-type": "text/plain",
"content-version": "vor",
"intended-application": "text-mining"
}
],
"member": "78",
"original-title": [],
"page": "100835",
"prefix": "10.1016",
"published": {
"date-parts": [
[
2023,
9
]
]
},
"published-print": {
"date-parts": [
[
2023,
9
]
]
},
"publisher": "Elsevier BV",
"reference": [
{
"key": "10.1016/j.lanwpc.2023.100835_bib1",
"series-title": "WHO coronavirus (COVID-19) dashboard",
"year": "2022"
},
{
"DOI": "10.1080/07853890.2022.2031274",
"article-title": "Emerging COVID-19 variants and their impact on SARS-CoV-2 diagnosis, therapeutics and vaccines",
"author": "Fernandes",
"doi-asserted-by": "crossref",
"first-page": "524",
"issue": "1",
"journal-title": "Ann Med",
"key": "10.1016/j.lanwpc.2023.100835_bib2",
"volume": "54",
"year": "2022"
},
{
"DOI": "10.1016/j.ijbiomac.2022.01.118",
"article-title": "Three waves changes, new variant strains, and vaccination effect against COVID-19 pandemic",
"author": "El-Shabasy",
"doi-asserted-by": "crossref",
"first-page": "161",
"journal-title": "Int J Biol Macromol",
"key": "10.1016/j.lanwpc.2023.100835_bib3",
"volume": "204",
"year": "2022"
},
{
"DOI": "10.1007/s11596-021-2395-1",
"article-title": "A comprehensive review of COVID-19 virology, vaccines, variants, and therapeutics",
"author": "Forchette",
"doi-asserted-by": "crossref",
"first-page": "1037",
"issue": "6",
"journal-title": "Curr Med Sci",
"key": "10.1016/j.lanwpc.2023.100835_bib4",
"volume": "41",
"year": "2021"
},
{
"DOI": "10.1056/EVIDoa2100044",
"article-title": "Randomized trial of molnupiravir or placebo in patients hospitalized with covid-19",
"author": "Arribas",
"doi-asserted-by": "crossref",
"issue": "2",
"journal-title": "NEJM Evidence",
"key": "10.1016/j.lanwpc.2023.100835_bib5",
"volume": "1",
"year": "2022"
},
{
"DOI": "10.3390/v13040628",
"article-title": "Neutralizing antibody therapeutics for COVID-19",
"author": "Hurt",
"doi-asserted-by": "crossref",
"first-page": "628",
"issue": "4",
"journal-title": "Viruses",
"key": "10.1016/j.lanwpc.2023.100835_bib6",
"volume": "13",
"year": "2021"
},
{
"DOI": "10.1056/NEJMoa2107934",
"article-title": "Early treatment for covid-19 with SARS-CoV-2 neutralizing antibody sotrovimab",
"author": "Gupta",
"doi-asserted-by": "crossref",
"first-page": "1941",
"issue": "21",
"journal-title": "N Engl J Med",
"key": "10.1016/j.lanwpc.2023.100835_bib7",
"volume": "385",
"year": "2021"
},
{
"DOI": "10.1093/cid/ciab494",
"article-title": "Outpatient treatment of severe acute respiratory syndrome coronavirus 2 infection to prevent coronavirus disease 2019 progression",
"author": "Cohen",
"doi-asserted-by": "crossref",
"first-page": "1717",
"issue": "9",
"journal-title": "Clin Infect Dis",
"key": "10.1016/j.lanwpc.2023.100835_bib8",
"volume": "73",
"year": "2021"
},
{
"DOI": "10.1038/s41586-021-04386-2",
"article-title": "Broadly neutralizing antibodies overcome SARS-CoV-2 Omicron antigenic shift",
"author": "Cameroni",
"doi-asserted-by": "crossref",
"first-page": "664",
"issue": "7898",
"journal-title": "Nature",
"key": "10.1016/j.lanwpc.2023.100835_bib9",
"volume": "602",
"year": "2022"
},
{
"DOI": "10.1186/s12929-022-00852-9",
"article-title": "A critical overview of current progress for COVID-19: development of vaccines, antiviral drugs, and therapeutic antibodies",
"author": "Kumari",
"doi-asserted-by": "crossref",
"first-page": "68",
"issue": "1",
"journal-title": "J Biomed Sci",
"key": "10.1016/j.lanwpc.2023.100835_bib10",
"volume": "29",
"year": "2022"
},
{
"DOI": "10.1016/j.biopha.2021.111313",
"article-title": "An update review of emerging small-molecule therapeutic options for COVID-19",
"author": "Tian",
"doi-asserted-by": "crossref",
"journal-title": "Biomed Pharmacother",
"key": "10.1016/j.lanwpc.2023.100835_bib11",
"volume": "137",
"year": "2021"
},
{
"DOI": "10.1016/j.ejmech.2020.112711",
"article-title": "The development of Coronavirus 3C-Like protease (3CL(pro)) inhibitors from 2010 to 2020",
"author": "Liu",
"doi-asserted-by": "crossref",
"journal-title": "Eur J Med Chem",
"key": "10.1016/j.lanwpc.2023.100835_bib12",
"volume": "206",
"year": "2020"
},
{
"DOI": "10.1080/1061186X.2020.1853736",
"article-title": "Potential inhibitors of SARS-CoV-2: recent advances",
"author": "Jamalipour Soufi",
"doi-asserted-by": "crossref",
"first-page": "349",
"issue": "4",
"journal-title": "J Drug Target",
"key": "10.1016/j.lanwpc.2023.100835_bib13",
"volume": "29",
"year": "2021"
},
{
"key": "10.1016/j.lanwpc.2023.100835_bib15",
"unstructured": "Medical devices (registration) -- Basic information of \"National Instrument Registration Permit 20203400064\". https://www.nmpa.gov.cn/datasearch/search-info.html?nmpa=aWQ9NWFiY2YzNjljMjQ4YmFiYzlmZjAyZGNlYzM3ZmQ1OWMmaXRlbUlkPWZmODA4MDgxODNjYWQ3NTAwMTgzY2I2NmZlNjkwMjg1."
},
{
"key": "10.1016/j.lanwpc.2023.100835_bib16",
"series-title": "Common Terminology criteria for adverse events (CTCAE), version 5.0",
"year": "2017"
},
{
"article-title": "Covid-19, hypokalaemia and the renin-angiotensin-aldosterone system",
"author": "Mandal",
"journal-title": "Ann Med Surg (Lond)",
"key": "10.1016/j.lanwpc.2023.100835_bib17",
"volume": "65",
"year": "2021"
},
{
"DOI": "10.1007/s10157-020-01996-4",
"article-title": "Hypokalemia in patients with COVID-19",
"author": "Alfano",
"doi-asserted-by": "crossref",
"first-page": "401",
"issue": "4",
"journal-title": "Clin Exp Nephrol",
"key": "10.1016/j.lanwpc.2023.100835_bib18",
"volume": "25",
"year": "2021"
},
{
"article-title": "Hypokalemia and clinical implications in patients with coronavirus disease 2019 (COVID-19)",
"author": "Chen",
"journal-title": "medRxiv",
"key": "10.1016/j.lanwpc.2023.100835_bib19",
"year": "2020"
},
{
"DOI": "10.1056/NEJMoa2118542",
"article-title": "Oral nirmatrelvir for high-risk, nonhospitalized adults with covid-19",
"author": "Hammond",
"doi-asserted-by": "crossref",
"first-page": "1397",
"issue": "15",
"journal-title": "N Engl J Med",
"key": "10.1016/j.lanwpc.2023.100835_bib20",
"volume": "386",
"year": "2022"
},
{
"DOI": "10.18433/jpps31870",
"article-title": "Safety and efficacy of remdesivir for the treatment of COVID-19: a systematic review and meta-analysis",
"author": "Tasavon Gholamhoseini",
"doi-asserted-by": "crossref",
"first-page": "237",
"journal-title": "J Pharm Pharm Sci",
"key": "10.1016/j.lanwpc.2023.100835_bib21",
"volume": "24",
"year": "2021"
},
{
"DOI": "10.1002/iid3.580",
"article-title": "The relationship between COVID-19 viral load and disease severity: a systematic review",
"author": "Dadras",
"doi-asserted-by": "crossref",
"issue": "3",
"journal-title": "Immun Inflamm Dis",
"key": "10.1016/j.lanwpc.2023.100835_bib22",
"volume": "10",
"year": "2022"
},
{
"article-title": "Correlation analysis between the viral load and the progression of COVID-19",
"author": "Chen",
"journal-title": "Comput Math Methods Med",
"key": "10.1016/j.lanwpc.2023.100835_bib23",
"volume": "2021",
"year": "2021"
},
{
"DOI": "10.3390/v13020304",
"article-title": "Relationships between viral load and the clinical course of COVID-19",
"author": "Tsukagoshi",
"doi-asserted-by": "crossref",
"first-page": "304",
"issue": "2",
"journal-title": "Viruses",
"key": "10.1016/j.lanwpc.2023.100835_bib24",
"volume": "13",
"year": "2021"
}
],
"reference-count": 23,
"references-count": 23,
"relation": {},
"resource": {
"primary": {
"URL": "https://linkinghub.elsevier.com/retrieve/pii/S2666606523001530"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Infectious Diseases",
"Psychiatry and Mental health",
"Geriatrics and Gerontology",
"Public Health, Environmental and Occupational Health",
"Obstetrics and Gynecology",
"Health Policy",
"Pediatrics, Perinatology and Child Health",
"Internal Medicine"
],
"subtitle": [],
"title": "Efficacy and safety of SIM0417 (SSD8432) plus ritonavir for COVID-19 treatment: a randomised, double-blind, placebo-controlled, phase 1b trial",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1016/elsevier_cm_policy",
"volume": "38"
}