Feb 19 |
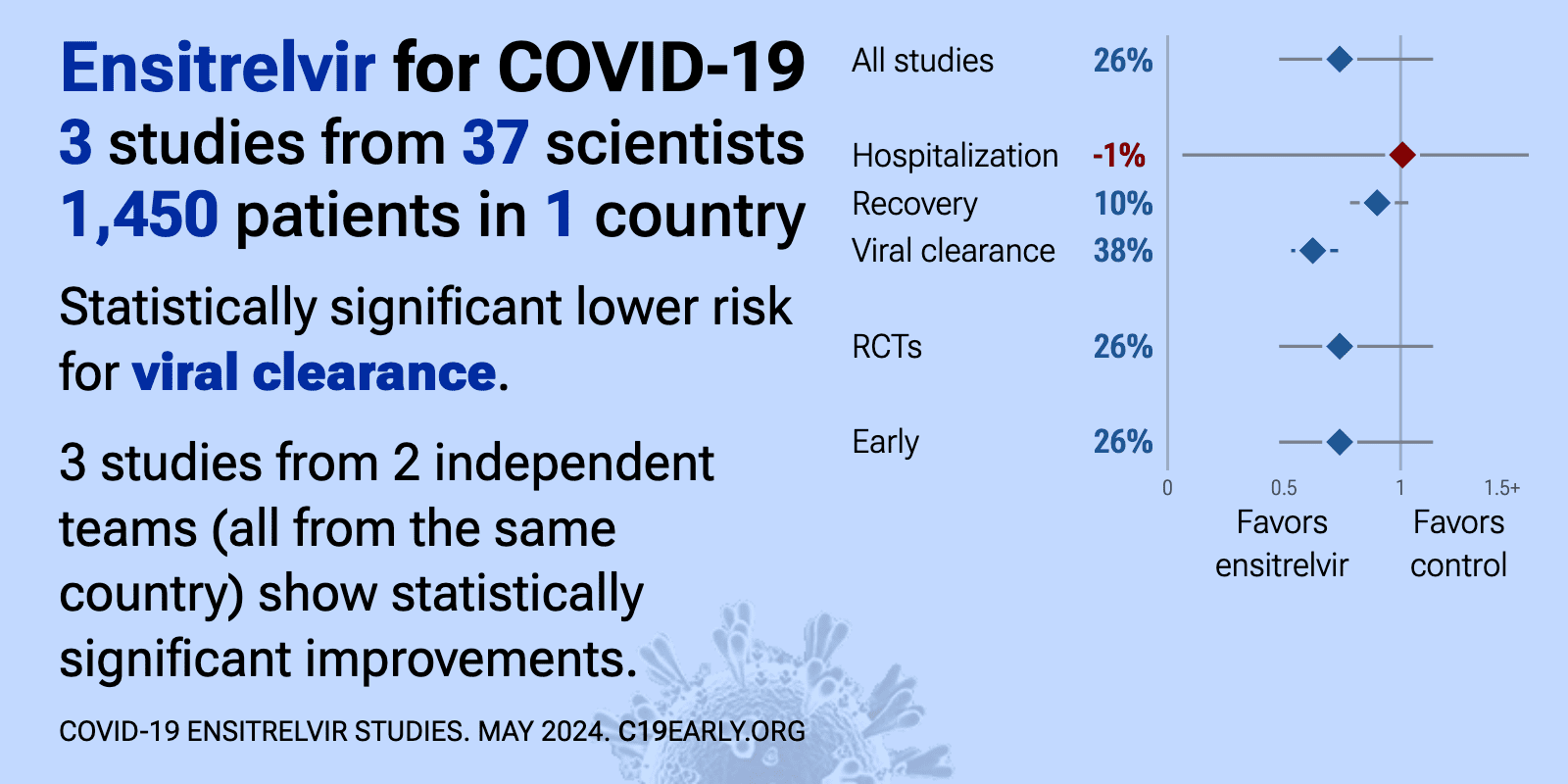
Ensitrelvir reduces COVID-19 risk: real-time meta-analysis of 8 studies (Version 14) | |
| Significantly lower risk is seen for hospitalization, recovery, cases, and viral clearance. 8 studies from 5 independent teams (all from the same country) show significant benefit. Meta-analysis using the most serious outcome reported sho.. | ||
Oct 14 2025 |
et al., Emerging Microbes & Infections, doi:10.1080/22221751.2025.2552716 | SARS-CoV-2 Mpro inhibitor ensitrelvir: asymmetrical cross-resistance with nirmatrelvir and emerging resistance hotspots |
| In vitro study showing that SARS-CoV-2 develops high-fitness resistance to ensitrelvir through Mpro mutations. Authors found asymmetrical cross-resistance, with ensitrelvir-resistant variants showing minimal cross-resistance to nirmatrelv.. | ||
Sep 29 2025 |
et al., BMC Infectious Diseases, doi:10.1186/s12879-025-11651-6 | Comparative effectiveness of antiviral treatment on household transmission of SARS-CoV-2: a retrospective cohort study using administrative data |
| Retrospective 5,398 married couples in Japan showing no significant difference in household transmission rates between molnupiravir, ensitrelvir, and paxlovid. Hospitalized patients receiving antivirals showed a trend toward lower transmi.. | ||
Jul 31 2025 |
et al., Infection, doi:10.1007/s15010-025-02582-0 | Efficacy and safety of Ensitrelvir in asymptomatic or mild to moderate COVID-19: a systematic review and meta-analysis of randomized controlled trials |
| Meta-analysis of 6 RCTs with 2,793 participants showing significantly lower viral load with ensitrelvir. However, treatment was associated with significant adverse effects including decreased HDL levels, elevated triglycerides, increased .. | ||
May 21 2025 |
et al., The Lancet Infectious Diseases, doi:10.1016/S1473-3099(25)00482-7 (date from preprint) | Antiviral efficacy of oral ensitrelvir versus oral ritonavir-boosted nirmatrelvir in COVID-19 |
| 8% faster recovery (p=0.006) and 49% improved viral clearance (p<0.0001). RCT 604 low-risk adults with early COVID-19 symptoms showing significantly improved SARS-CoV-2 viral clearance with both ensitrelvir and paxlovid. Inclusion criteria selected for low-risk patients with high viral loads which may not gener.. | ||
Apr 30 2025 |
et al., Journal of Infection and Chemotherapy, doi:10.1016/j.jiac.2025.102669 | The time to return-to-work in healthcare workers with COVID-19 treated with ensitrelvir, a novel oral inhibitor of 3C-like protease of SARS-CoV-2: An observational study utilizing pre-existing data from a single hospital |
| 10% faster recovery (p=0.02). Retrospective 102 healthcare workers in Japan showing shorter time to return-to-work with ensitrelvir treatment for COVID-19. | ||
Mar 9 2025 |
et al., CROI 2025 | Ensitrelvir to Prevent COVID-19 in Households: SCORPIO-PEP Phase III Placebo-Controlled Trial Results |
| 68% fewer symptomatic cases (p<0.0001). RCT 2,041 household contacts showing significantly lower symptomatic COVID-19 cases with ensitrelvir post-exposure prophylaxis. | ||
Feb 17 2025 |
et al., Clinical Infectious Diseases, doi:10.1093/cid/ciaf029 | Ensitrelvir for the Treatment of Nonhospitalized Adults with COVID-19: Results from the SCORPIO-HR, Phase 3, Randomized, Double-blind, Placebo-Controlled Trial |
| 203% higher hospitalization (p=0.37), 5% faster recovery (p=0.14), and 19% improved viral clearance (p=0.02). RCT 2,093 outpatients with mild-to-moderate COVID-19 showing improved viral clearance but no significant difference in time to symptom resolution with ensitrelvir. Participants were randomized to receive ensitrelvir or placebo within five.. | ||
Aug 12 2024 |
et al., The Journal of Infectious Diseases, doi:10.1093/infdis/jiae385 | Persistence of an infectious form of SARS-CoV-2 post protease inhibitor treatment of permissive cells in vitro |
| In vitro study showing the persistence of an infectious form of SARS-CoV-2 after treatment with 3CLpro inhibitors nirmatrelvir and ensitrelvir, which may explain the rebound often seen with paxlovid. 3CLpro is crucial for processing viral.. | ||
Jul 6 2024 |
et al., Quantitative Biology, doi:10.1002/qub2.60 | Assessing the inhibition efficacy of clinical drugs against the main proteases of SARS‐CoV‐2 variants and other coronaviruses |
| In vitro study showing that leritrelvir and GC376 remained effective against some nirmatrelvir- and ensitrelvir-resistant Mpro mutants. Leritrelvir showed better broad-spectrum activity against other pathogenic coronaviruses compared to e.. | ||
Jun 28 2024 |
et al., Infectious Diseases and Therapy, doi:10.1007/s40121-024-01010-4 | Real-World Effectiveness of Ensitrelvir in Reducing Severe Outcomes in Outpatients at High Risk for COVID-19 |
| 24% higher mortality (p=0.84), 158% higher ventilation (p=0.2), 36% lower need for oxygen therapy (p=0.33), and 37% lower hospitalization (p=0.02). Retrospective 167,310 high-risk COVID-19 outpatients in Japan showing significantly lower hospitalization with ensitrelvir treatment. | ||
Jun 24 2024 |
et al., Current Clinical Microbiology Reports, doi:10.1007/s40588-024-00229-6 | SARS-CoV-2 Resistance to Small Molecule Inhibitors |
| Review of resistance mutations in SARS-CoV-2 3CLpro and nsp12 that could reduce efficacy of antiviral therapies including nirmatrelvir, ensitrelvir, remdesivir, and favipiravir. Authors identify 39 single mutations across 17 critical resi.. | ||
Feb 29 2024 |
et al., Antiviral Research, doi:10.1016/j.antiviral.2024.105852 | Prophylactic effect of ensitrelvir in mice infected with SARS-CoV-2 |
| Mouse study showing protective effects against SARS-CoV-2 infection in aged mice with the 3CL protease inhibitor ensitrelvir. A single subcutaneous dose of ensitrelvir at 64, 96, or 128 mg/kg given 24 hours before a lethal SARS-CoV-2 chal.. | ||
Feb 29 2024 |
et al., Antiviral Research, doi:10.1016/j.antiviral.2024.105814 | Generation and evaluation of protease inhibitor-resistant SARS-CoV-2 strains |
| In vitro and animal study showing that SARS-CoV-2 can develop resistance to nirmatrelvir and ensitrelvir. Authors generated resistant viral strains through repeated passaging with both drugs. For nirmatrelvir, they identified three differ.. | ||
Feb 6 2024 |
et al., Cureus, doi:10.7759/cureus.61048 (date from preprint) | Real-World Efficacy of Ensitrelvir in Hospitalized Patients With COVID-19 in Japan: A Retrospective Observational Study |
| Retrospective 154 hospitalized COVID-19 patients in Japan showing faster viral clearance and shorter hospitalization with ensitrelvir treatment compared to remdesivir or molnupiravir. There was no significant difference for fever resoluti.. | ||
Dec 31 2023 |
, E., Aging and disease, doi:10.14336/AD.2023.0318 | Mitigating COVID-19 Mortality and Morbidity in China's Aging Population: A Focus on Available Medications and Future Developments |
| Review focusing on 3CL protease inhibitors. First generation inhibitors like paxlovid and simnotrelvir require boosting with ritonavir, which can cause drug-drug interactions and other issues. Second generation inhibitors like ensitrelvir.. | ||
Nov 30 2023 |
et al., iScience, doi:10.1016/j.isci.2023.108147 | Antiviral efficacy against and replicative fitness of an XBB.1.9.1 clinical isolate |
| In vitro and animal study showing that the SARS-CoV-2 omicron subvariant XBB.1.9.1 has similar antigenicity, antiviral susceptibility, and replicative ability compared to XBB.1.5. Casirivimab, imdevimab, tixagevimab, cilgavimab, sotrovima.. | ||
Aug 20 2023 |
et al., npj Antimicrobials and Resistance, doi:10.1038/s44259-023-00009-0 | Rapid resistance profiling of SARS-CoV-2 protease inhibitors |
| In vitro study of SARS-CoV-2 main protease (Mpro) variants showing distinct resistance profiles for the Mpro inhibitors nirmatrelvir, ensitrelvir, and FB2001. Results show significant resistance for multiple variants for nirmatrelvir and .. | ||
Jul 13 2023 |
et al., JAMA Network Open, doi:10.1001/jamanetworkopen.2023.54991 (date from preprint) | Efficacy and Safety of 5-Day Oral Ensitrelvir for Patients With Mild to Moderate COVID-19: The SCORPIO-SR Randomized Clinical Trial |
| 11% faster recovery (p=0.3) and 49% improved viral clearance (p=0.001). RCT 1,821 COVID-19 outpatients in Japan, Vietnam, and South Korea, showing improved viral clearance and improved recovery (significant for patients treated within 3 days of onset) with ensitrelvir. Only 2 hospitalizations were reported, w.. | ||
Mar 31 2023 |
et al., Science Advances, doi:10.1126/sciadv.ade8778 | Transmissible SARS-CoV-2 variants with resistance to clinical protease inhibitors |
| Analysis of naturally occurring SARS-CoV-2 main protease variants for resistance to nirmatrelvir and ensitrelvir. Authors identified multiple single amino acid mutations that confer significant resistance to these drugs. Phylogenetic anal.. | ||
Feb 10 2023 |
et al., Journal of Antimicrobial Chemotherapy, doi:10.1093/jac/dkad027 | Efficacy comparison of 3CL protease inhibitors ensitrelvir and nirmatrelvir against SARS-CoV-2 in vitro and in vivo |
| In vitro and animal study comparing nirmatrelvir and ensitrelvir, showing similar efficacy in vitro, and equal or better efficacy of ensitrelvir in vivo (with similar unbound-drug plasma concentrations). | ||
Dec 7 2022 |
et al., Clinical Infectious Diseases, doi:10.1093/cid/ciac933 | Efficacy and safety of ensitrelvir in patients with mild-to-moderate COVID-19: the phase 2b part of a randomized, placebo-controlled, phase 2/3 study |
| 9% improved recovery (p=0.28) and 32% faster viral clearance (p=0.0001). RCT 428 COVID-19 patients in Japan showing faster viral clearance and improved recovery with ensitrelvir. | ||
May 17 2022 |
et al., medRxiv, doi:10.1101/2022.05.17.22275027 | A Randomized Phase 2/3 Study of Ensitrelvir, a Novel Oral SARS-CoV-2 3C-like Protease Inhibitor, in Japanese Patients With Mild-to-Moderate COVID-19 or Asymptomatic SARS-CoV-2 Infection: Results of the Phase 2a Part |
| 45% improved viral clearance (p=0.002). RCT 69 patients in in Japan, showing faster viral clearance with ensitrelvir. 5-day ensitrelvir (375mg on day 1 followed by 125 mg daily or 750mg on day 1 followed by 250mg daily). | ||
References