Ensitrelvir for the Treatment of Nonhospitalized Adults with COVID-19: Results from the SCORPIO-HR, Phase 3, Randomized, Double-blind, Placebo-Controlled Trial
et al., Clinical Infectious Diseases, doi:10.1093/cid/ciaf029, SCORPIO-HR, Feb 2025
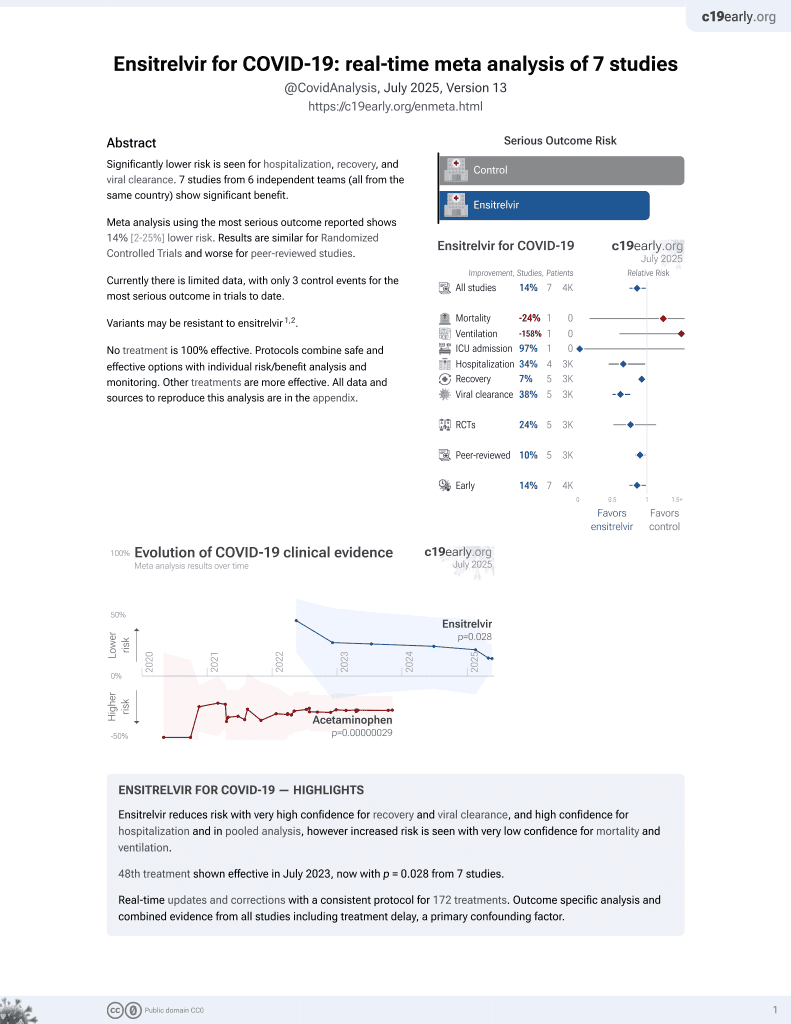
50th treatment shown to reduce risk in
July 2023, now with p = 0.015 from 8 studies.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
RCT 2,093 outpatients with mild-to-moderate COVID-19 showing improved viral clearance but no significant difference in time to symptom resolution with ensitrelvir. Participants were randomized to receive ensitrelvir or placebo within five days of symptom onset, with the primary analysis focusing on those treated within three days. A mid-trial protocol change excluded participants treated more than three days post-symptom onset from the primary analysis.
|
risk of hospitalization, 203.2% higher, RR 3.03, p = 0.37, treatment 3 of 1,037 (0.3%), control 1 of 1,048 (0.1%).
|
|
recovery time, 4.6% lower, relative time 0.95, p = 0.14, treatment 945, control 943.
|
|
risk of no viral clearance, 18.7% lower, RR 0.81, p = 0.02, treatment 175 of 882 (19.8%), control 215 of 881 (24.4%), NNT 22, day 8.
|
|
risk of no viral clearance, 16.1% lower, RR 0.84, p < 0.001, treatment 420 of 835 (50.3%), control 517 of 862 (60.0%), NNT 10, day 4.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Luetkemeyer et al., 17 Feb 2025, Double Blind Randomized Controlled Trial, placebo-controlled, multiple countries, peer-reviewed, 22 authors, SCORPIO-HR trial.
Ensitrelvir for the Treatment of Nonhospitalized Adults with COVID-19: Results from the SCORPIO-HR, Phase 3, Randomized, Double-blind, Placebo-Controlled Trial
doi:10.1093/cid/ciaf029/8017725
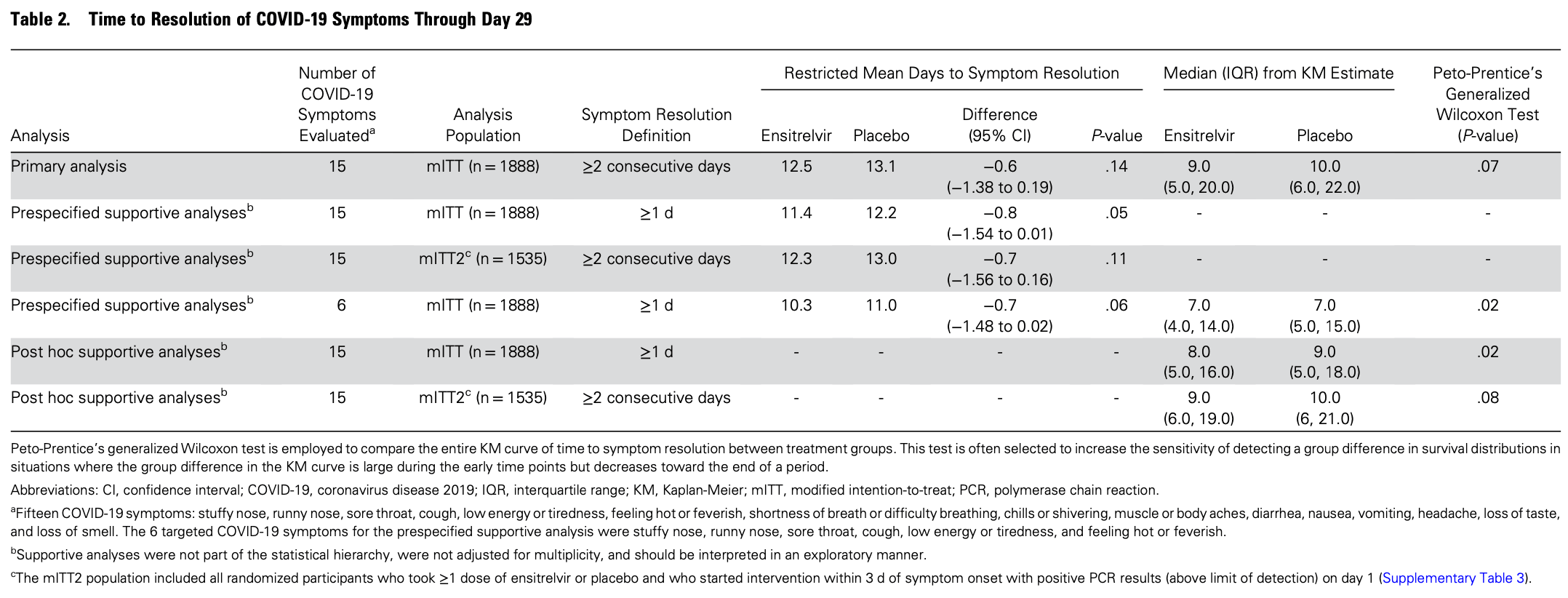
Background. Ensitrelvir, a severe acute respiratory syndrome coronavirus-2 main protease inhibitor, has demonstrated clinical and virologic efficacy in previous studies. Methods. In this global phase 3 trial, nonhospitalized adults with mild-to-moderate coronavirus disease 2019 (COVID-19) and symptom onset within 5 days were randomized (1:1) to receive once-daily ensitrelvir (375 mg day 1, 125 mg days 2-5) or blinded matching placebo. The primary endpoint was the restricted mean time to sustained (≥2 days) resolution of 15 COVID-19 symptoms, recorded in participant daily diaries, through day 29 in participants starting treatment within 3 days after symptom onset. Virologic efficacy and safety were assessed. Results. Of 2093 participants, 1888 started treatment within 3 days after symptom onset. Mean time to symptom resolution was 12.5 and 13.1 days with ensitrelvir and placebo, respectively (difference, -0.6 days; 95% confidence interval, -1.38 to 0.19; P = .14). On day 4, ensitrelvir reduced least-squares mean RNA by 0.72 log 10 copies/mL more than placebo (95% confidence interval, 0.55-0.90). Among those with positive viral cultures at enrollment, 274/287 (95.5%) ensitrelvir-treated versus 210/280 (75.0%) placebo-treated participants had negative cultures on day 4. RNA rebound was similar (<1.5%) between groups. The proportion of participants with ≥1 adverse event was similar with ensitrelvir (61.5%) and placebo (60.6%). No treatment-related serious adverse events or deaths occurred. Three (0.3%) ensitrelvir-treated and 1 (0.1%) placebo-treated participants had COVID-19-related hospitalizations by day 29. Conclusions. Despite the evidence of antiviral activity with ensitrelvir, this trial did not demonstrate a significant difference in time to sustained symptom resolution.
Peto-Prentice's generalized Wilcoxon test is employed to compare the entire KM curve of time to symptom resolution between treatment groups. This test is often selected to increase the sensitivity of detecting a group difference in survival distributions in situations where the group difference in the KM curve is large during the early time points but decreases toward the end of a period. Abbreviations: CI, confidence interval; COVID-19, coronavirus disease 2019; IQR, interquartile range; KM, Kaplan-Meier; mITT, modified intention-to-treat; PCR, polymerase chain reaction. a Fifteen COVID-19 symptoms: stuffy nose, runny nose, sore throat, cough, low energy or tiredness, feeling hot or feverish, shortness of breath or difficulty breathing, chills or shivering, muscle or body aches, diarrhea, nausea, vomiting, headache, loss of taste, and loss of smell. The 6 targeted COVID-19 symptoms for the prespecified supportive analysis were stuffy nose, runny nose, sore throat, cough, low energy or tiredness, and feeling hot or feverish. b Supportive analyses were not part of the statistical hierarchy, were not adjusted for multiplicity, and should be interpreted in an exploratory manner. c The mITT2 population included all randomized participants who took ≥1 dose of ensitrelvir or placebo and who started intervention within 3 d of symptom onset with positive PCR results (above limit of detection) on day 1 (Supplementary Table 3 ).
Supplementary Data Supplementary materials are..
References
Acquisition, Anwar Santoso, Armstrong, Driver, Russell et al., ) and funded by Shionogi. The authors acknowledge Katia Russionello from Shionogi Inc. for her role in safety monitoring and analysis and Bryan Kosten from Shionogi Inc. for his role in study operations and oversight
Butler, Hobbs, Gbinigie, Molnupiravir plus usual care versus usual care alone as early treatment for adults with COVID-19 at increased risk of adverse outcomes (PANORAMIC): an open-label, platform-adaptive randomised controlled trial, Lancet
Cao, Jian, Wang, Imprinted SARS-CoV-2 humoral immunity induces convergent Omicron RBD evolution, Nature
Cao, Wang, Lu, Oral simnotrelvir for adult patients with mild-to-moderate COVID-19, N Engl J Med
Cvancara, Baertsch, Lehmann, Postmarketing reporting of Paxlovid-related dysgeusia: a real-world pharmacovigilance study, Otolaryngol Head Neck Surg
Degli-Angeli, Dragavon, Huang, Validation and verification of the Abbott RealTime SARS-CoV-2 assay analytical and clinical performance, J Clin Virol
Edelstein, Boucau, Uddin, SARS-CoV-2 virologic rebound with nirmatrelvir-ritonavir therapy: an observational study, Ann Intern Med
Feikin, Higdon, Abu-Raddad, Duration of effectiveness of vaccines against SARS-CoV-2 infection and COVID-19 disease: results of a systematic review and meta-regression, Lancet
Guan, Puenpatom, Johnson, Impact of molnupiravir treatment on patient-reported COVID-19 symptoms in the phase 3 MOVe-OUT trial: a randomized, placebo-controlled trial, Clin Infect Dis
Hammond, Fountaine, Yunis, Nirmatrelvir for vaccinated or unvaccinated adult outpatients with COVID-19, N Engl J Med
Hammond, Leister-Tebbe, Gardner, Oral nirmatrelvir for high-risk, nonhospitalized adults with COVID-19, N Engl J Med
Hammond, Leister-Tebbe, Gardner, Sustained alleviation and resolution of targeted COVID-19 symptoms with nirmatrelvir/ritonavir versus placebo, Open Forum Infect Dis
Kawashima, Matsui, Adachi, Ensitrelvir is effective against SARS-CoV-2 3CL protease mutants circulating globally, Biochem Biophys Res Commun
Kozlov, COVID drug Paxlovid was hailed as a game-changer. What happened?, Nature
Lagevrio™, Fact sheet for healthcare providers
Lazarus, Wyka, White, A survey of COVID-19 vaccine acceptance across 23 countries in 2022, Nat Med
Marzolini, Kuritzkes, Marra, Recommendations for the management of drug-drug interactions between the COVID-19 antiviral nirmatrelvir/ritonavir (Paxlovid) and comedications, Clin Pharmacol Ther
Menegale, Manica, Zardini, Evaluation of waning of SARS-CoV-2 vaccine-induced immunity: a systematic review and meta-analysis, JAMA Netw Open
Mukae, Yotsuyanagi, Ohmagari, A randomized phase 2/3 study of ensitrelvir, a novel oral SARS-CoV-2 3C-like protease inhibitor, in Japanese patients with mild-to-moderate COVID-19 or asymptomatic SARS-CoV-2 infection: results of the phase 2a part, Antimicrob Agents Chemother
Mukae, Yotsuyanagi, Ohmagari, Efficacy and safety of ensitrelvir in patients with mild-to-moderate coronavirus disease 2019: the phase 2b part of a randomized, placebo-controlled, phase 2/3 study, Clin Infect Dis
Sasaki, Tabata, Kishimoto, S-217622, a SARS-CoV-2 main protease inhibitor, decreases viral load and ameliorates COVID-19 severity in hamsters, Sci Transl Med
Unoh, Uehara, Nakahara, Discovery of S-217622, a noncovalent oral SARS-CoV-2 3CL protease inhibitor clinical candidate for treating COVID-19, J Med Chem
Uraki, Ito, Kiso, Antiviral and bivalent vaccine efficacy against an omicron XBB.1.5 isolate, Lancet Infect Dis
Uraki, Kiso, Iida, Characterization and antiviral susceptibility of SARS-CoV-2 Omicron BA.2, Nature
Yotsuyanagi, Ohmagari, Doi, Efficacy and safety of 5-day oral ensitrelvir for patients with mild to moderate COVID-19: the SCORPIO-SR randomized clinical trial, JAMA Netw Open
Yotsuyanagi, Ohmagari, Doi, Prevention of post COVID-19 condition by early treatment with ensitrelvir in the phase 3 SCORPIO-SR trial, Antiviral Res
Zhao, Bashiri, Ziora, Toth, Skwarczynski, COVID-19 variants and vaccine development, Viruses