Feb 19 |
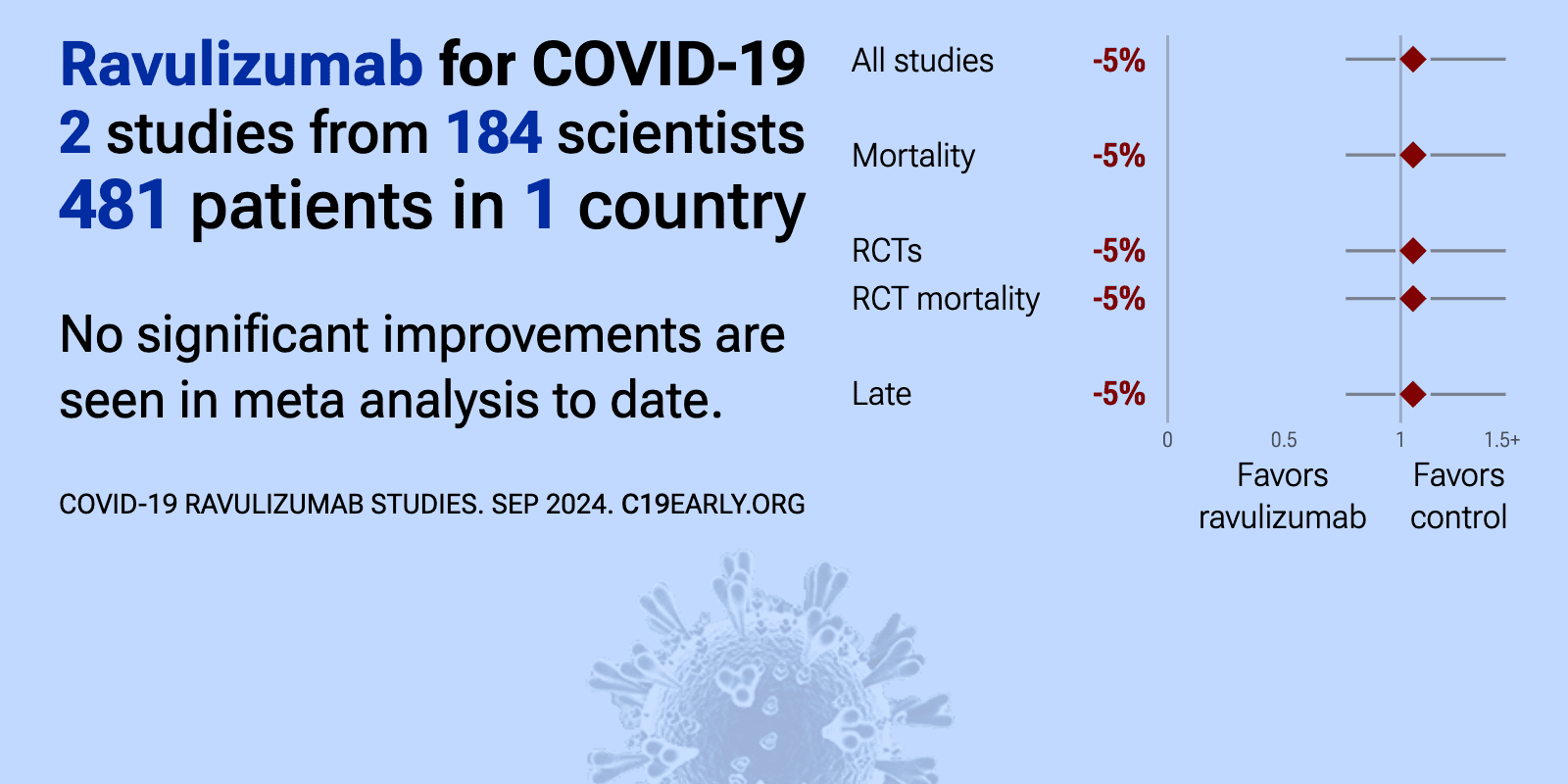
Meta-analysis of ravulizumab studies | |
| Meta-analysis of ravulizumab studies | ||
Dec 31 2023 |
et al., The Lancet Respiratory Medicine, doi:10.1016/S2213-2600(23)00376-4 | Efficacy and safety of baricitinib or ravulizumab in adult patients with severe COVID-19 (TACTIC-R): a randomised, parallel-arm, open-label, phase 4 trial |
| 1% lower mortality (p=0.98), 53% higher progression (p=0.13), 24% higher need for oxygen therapy (p=0.57), and 3% lower hospital discharge (p=0.86). RCT 417 hospitalized COVID-19 patients in the UK showing no significant difference in a composite primary endpoint (time to death, invasive mechanical ventilation, extracorporeal membrane oxygenation, cardiovascular organ support, or rena.. | ||
Mar 20 2023 |
et al., The Lancet Respiratory Medicine, doi:10.1016/S2213-2600(23)00082-6 | Intravenous ravulizumab in mechanically ventilated patients hospitalised with severe COVID-19: a phase 3, multicentre, open-label, randomised controlled trial |
| 8% higher mortality (p=0.76). RCT 201 mechanically ventilated patients with severe COVID-19 showing no significant difference in mortality with ravulizumab. The study was terminated early due to futility. | ||