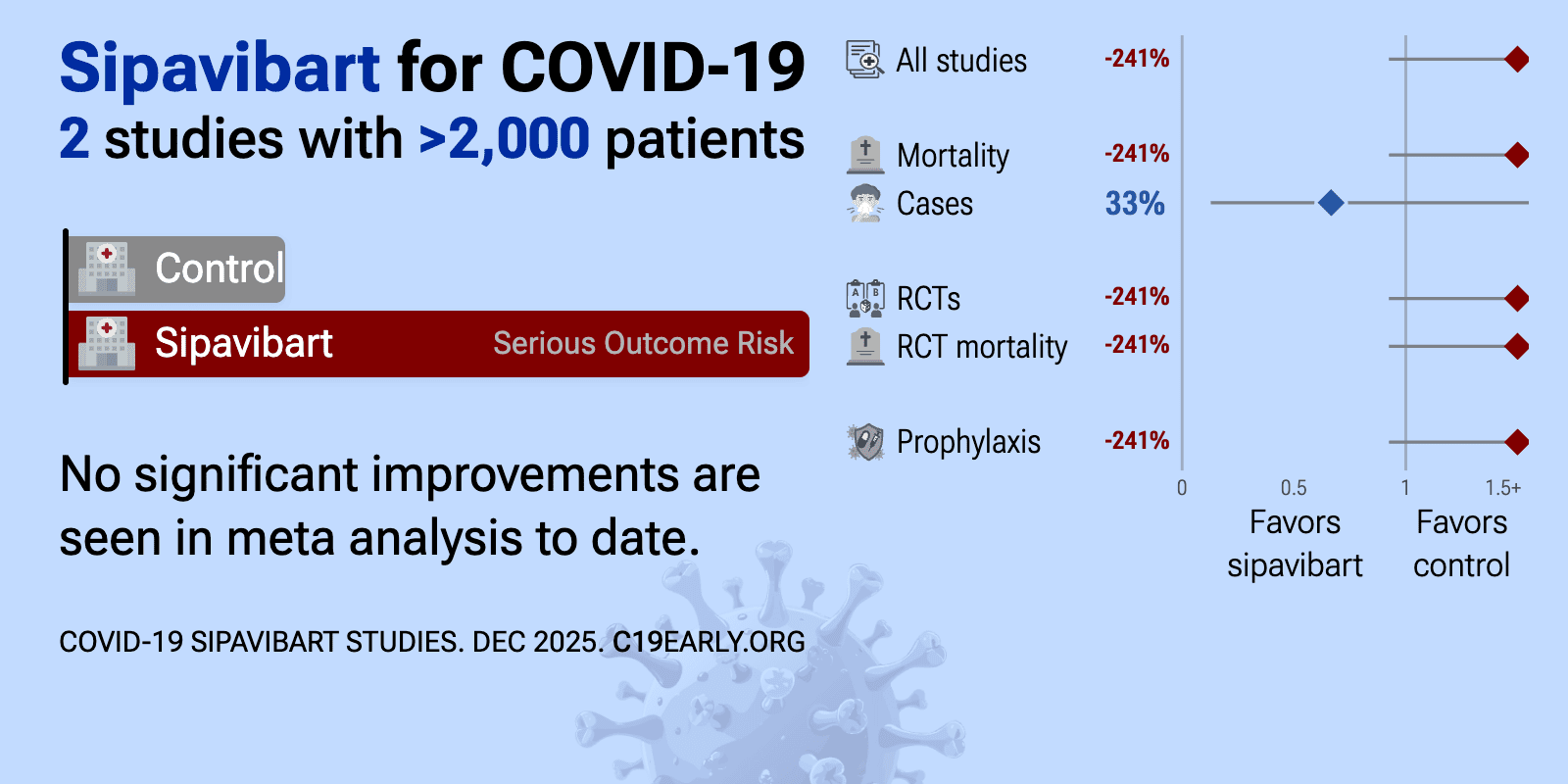
Feb 21 |
Meta-analysis of sipavibart studies | |
| Meta-analysis of sipavibart studies | ||
Jul 31 2025 |
et al., The Lancet Infectious Diseases, doi:10.1016/S1473-3099(24)00804-1 | Efficacy and safety of sipavibart for prevention of COVID-19 in individuals who are immunocompromised (SUPERNOVA): a randomised, controlled, double-blind, phase 3 trial |
| 236% higher mortality (p=0.09), 1% lower hospitalization (p=1), and 32% fewer cases (p=0.0005). RCT 3,349 immunocompromised patients showing lower symptomatic COVID-19 with sipavibart versus a combined comparator group. There was no significant difference for severe COVID-19 or hospitalization. All-cause mortality and adverse events.. | ||
May 26 2025 |
et al., Infectious Diseases, doi:10.1080/23744235.2025.2509011 | Dynamics of SARS-CoV-2 variants and mutations in Central Sweden between 2023 and 2024 and their potential implications on monoclonal antibodies pemivibart and sipavibart as PrEP in the region |
| Analysis of SARS-CoV-2 variants and mutations in central Sweden from October 2023 to October 2024, showing the rise of resistance mutations that likely render monoclonal antibodies sipavibart and pemivibart ineffective. | ||
Sep 30 2024 |
et al., Pathogens and Immunity, doi:10.20411/pai.v10i1.752 | Escape of SARS-CoV-2 Variants KP.1.1, LB.1, and KP.3.3 From Approved Monoclonal Antibodies |
| In vitro study showing significant escape of SARS-CoV-2 variants KP.1.1, LB.1, and KP.3.3 with monoclonal antibodies pemivibart (VYD222) and sipavibart (AZD3152). Sipavibart lost antiviral efficacy, while pemivibart maintained reduced act.. | ||
Aug 14 2024 |
et al., Human Vaccines & Immunotherapeutics, doi:10.1080/21645515.2024.2387221 | Characteristics of the first immunocompromised patients to receive sipavibart as an early access treatment for COVID-19 pre-exposure prophylaxis in France |
| Retrospective 47 immunocompromised patients in France showing no adverse events with sipavibart, an investigational long-acting monoclonal antibody, as COVID-19 pre-exposure prophylaxis. The patients had various immunosuppressive conditio.. | ||
May 17 2024 |
, NCT06057064 | A Phase II Randomized, Double-blind Study to Evaluate the Safety, Neutralizing Activity and Efficacy of AZD3152 for Pre-exposure Prophylaxis of COVID-19 in Participants Having an Increased Risk for Inadequate Response to Active Immunization (NOVELLA) |
| 267% higher mortality (p=1) and 33% fewer symptomatic cases (p=0.64). RCT 116 patients in Russia, showing improved nAb titers with sipavibart prophylaxis, but no significant difference for mortality or symptomatic cases. | ||
References
Focosi et al., Analysis of SARS-CoV-2 mutations associated with resistance to therapeutic monoclonal antibodies that emerge after treatment, Drug Resistance Updates, doi:10.1016/j.drup.2023.100991.
Leducq et al., Spike protein genetic evolution in patients at high-risk of severe COVID-19 treated by monoclonal antibodies, The Journal of Infectious Diseases, doi:10.1093/infdis/jiad523.
Bruhn et al., Somatic hypermutation shapes the viral escape profile of SARS-CoV-2 neutralising antibodies, eBioMedicine, doi:10.1016/j.ebiom.2025.105770.
Ngiam et al., Early administration of neutralising monoclonal antibodies and post-acute sequelae of COVID-19, International Journal of Infectious Diseases, doi:10.1016/j.ijid.2026.108435.
Choudhary et al., Emergence of SARS-CoV-2 Resistance with Monoclonal Antibody Therapy, medRxiv, doi:10.1101/2021.09.03.21263105.
Günther et al., Variant-specific humoral immune response to SARS-CoV-2 escape mutants arising in clinically severe, prolonged infection, medRxiv, doi:10.1101/2024.01.06.24300890.
Casadevall et al., Single monoclonal antibodies should not be used for COVID-19 therapy: a call for antiviral stewardship, Clinical Infectious Diseases, doi:10.1093/cid/ciae408.