A Phase II Randomized, Double-blind Study to Evaluate the Safety, Neutralizing Activity and Efficacy of AZD3152 for Pre-exposure Prophylaxis of COVID-19 in Participants Having an Increased Risk for Inadequate Response to Active Immunization (NOVELLA)
, NCT06057064, NOVELLA, NCT06057064, May 2024
RCT 116 patients in Russia, showing improved nAb titers with sipavibart prophylaxis, but no significant difference for mortality or symptomatic cases.
|
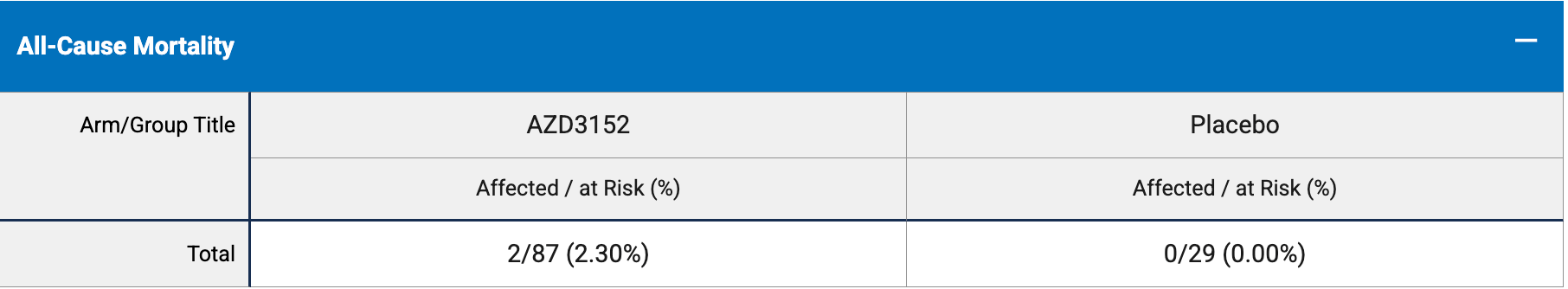
risk of death, 266.7% higher, RR 3.67, p = 1.00, treatment 2 of 87 (2.3%), control 0 of 29 (0.0%), continuity correction due to zero event (with reciprocal of the contrasting arm).
|
|
risk of symptomatic case, 33.3% lower, RR 0.67, p = 0.64, treatment 4 of 87 (4.6%), control 2 of 29 (6.9%), NNT 44.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
AstraZeneca et al., 17 May 2024, Double Blind Randomized Controlled Trial, placebo-controlled, Russia, preprint, 1 author, trial NCT06057064 (history) (NOVELLA).
Contact: information.center@astrazeneca.com.