Deuremidevir was adopted
in 2 countries.
Feb 23 |
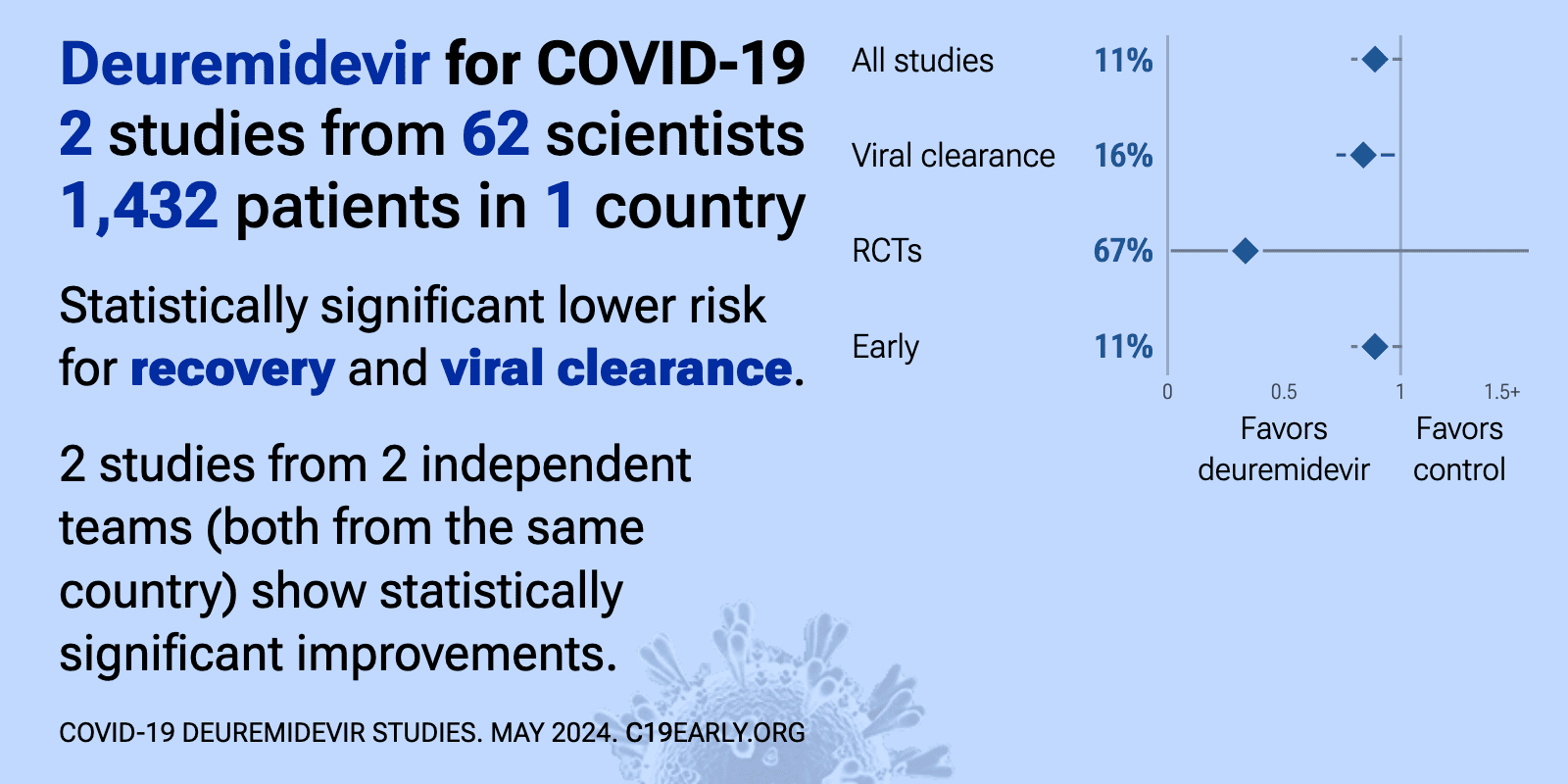
Deuremidevir for COVID-19: real-time meta-analysis of 2 studies | |
| Significantly lower risk is seen for recovery and viral clearance. 2 studies from 2 independent teams (both from the same country) show significant benefit. Meta-analysis using the most serious outcome reported shows 11% [-1‑21.. | ||
Mar 13 2024 |
et al., JAMA Network Open, doi:10.1001/jamanetworkopen.2024.1765 | COVID-19 Rebound After VV116 vs Nirmatrelvir-Ritonavir Treatment |
| RCT showing high rates of viral and symptom rebound with both paxlovid and deuremidevir (VV116). There are multiple potential reasons, with one being the highly specific targets within viral replication (Mpro and RdRp respectively). Paxlo.. | ||
Jan 18 2024 |
et al., International Journal of Antimicrobial Agents, doi:10.1016/j.ijantimicag.2024.107096 | Small-molecule antivirals treatment for COVID-19: A systematic review and network meta-analysis |
| Systematic review and network meta-analysis of 160 studies involving over 900,000 COVID-19 patients assessing the efficacy and safety of small-molecule antivirals. For VV116, significant benefit was found for viral clearance. | ||
Nov 30 2023 |
et al., The Lancet Infectious Diseases, doi:10.1016/S1473-3099(23)00577-7 | Oral VV116 versus placebo in patients with mild-to-moderate COVID-19 in China: a multicentre, double-blind, phase 3, randomised controlled study |
| 15% faster recovery (p=0.01) and 24% improved viral clearance (p=0.006). RCT 1,369 patients with mild-to-moderate COVID-19 showing significantly faster time to sustained clinical symptom resolution (median 10.9 days vs 12.9 days) with VV116 compared to placebo. VV116 was given as 0.6g BID day 1 followed by 0.3.. | ||
Nov 23 2023 |
et al., Viruses, doi:10.3390/v15122295 | Remdesivir Derivative VV116 Is a Potential Broad-Spectrum Inhibitor of Both Human and Animal Coronaviruses |
| In vitro study showing the remdesivir derivative VV116 exhibits broad-spectrum inhibition against human coronaviruses (HCoV-NL63, HCoV-229E, HCoV-OC43) and animal coronaviruses (MHV, FIPV, FECV, CCoV) without cytotoxicity. Through time-of.. | ||
Jul 31 2023 |
et al., Progress in Pharmaceutical Sciences, doi:10.20053/j.issn1001-5094.2023.07.003 | Clinical Research and Development of Deuremidevir Hydrobromide Tablets for the Treatment of COVID-19 |
| Review of the research and development of deuremidevir, an oral nucleoside analog targeting the RNA-dependent RNA polymerase (RdRp) of SARS-CoV-2, for the treatment of COVID-19. Deuremidevir was approved for marketing in China in January .. | ||
Jul 7 2023 |
et al., Medicine, doi:10.1097/MD.0000000000034105 | The safety and efficacy of oral antiviral drug VV116 for treatment of COVID-19: A systematic review |
| Systematic review of 3 VV116 studies with 1,044 participants. Authors conclude that VV116 had minimal adverse events and was not inferior in symptomatic alleviation when compared with paxlovid. Authors note that more research into the saf.. | ||
Dec 28 2022 |
et al., New England Journal of Medicine, doi:10.1056/NEJMoa2208822 | VV116 versus Nirmatrelvir–Ritonavir for Oral Treatment of Covid-19 |
| RCT 771 hospitalized patients with mild-to-moderate COVID-19 and high risk of progression showing non-inferior time to sustained clinical recovery with 5 days of VV116 compared to 5 days of paxlovid. No deaths or progression to severe dis.. | ||
Jun 2 2022 |
et al., Emerging Microbes & Infections, doi:10.1080/22221751.2022.2078230 | An open, prospective cohort study of VV116 in Chinese participants infected with SARS-CoV-2 omicron variants |
| 11% faster viral clearance (p=0.07). Prospective 136 hospitalized mild to moderate COVID-19 patients in China, showing faster viral clearance with VV116 treatment. 60 patients received VV116 300mg twice daily for 5 days plus standard care, while 76 patients received only sta.. | ||
Mar 16 2022 |
et al., Acta Pharmacologica Sinica, doi:10.1038/s41401-022-00895-6 | Safety, tolerability, and pharmacokinetics of VV116, an oral nucleoside analog against SARS-CoV-2, in Chinese healthy subjects |
| Phase 1 study of the safety, tolerability, and pharmacokinetics of VV116, an oral nucleoside analog prodrug, in healthy subjects. A single ascending dose study, multiple ascending dose study, and food effect study were conducted sequentia.. | ||