An open, prospective cohort study of VV116 in Chinese participants infected with SARS-CoV-2 omicron variants
et al., Emerging Microbes & Infections, doi:10.1080/22221751.2022.2078230, NCT05242042, Jun 2022
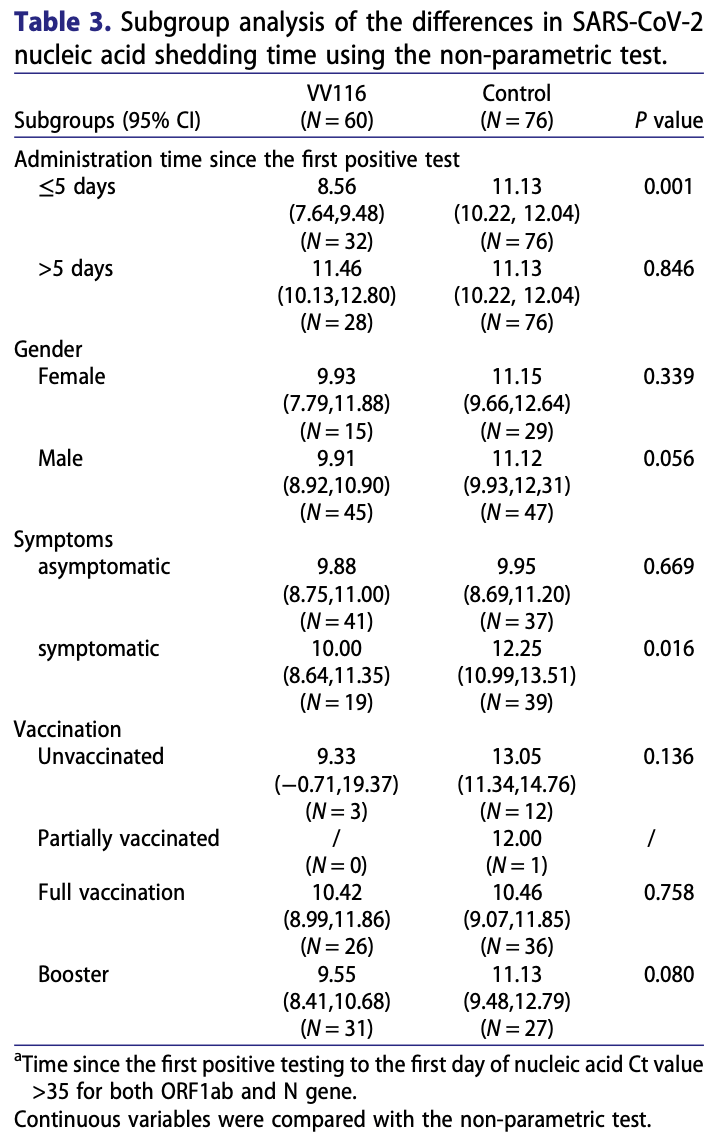
Prospective 136 hospitalized mild to moderate COVID-19 patients in China, showing faster viral clearance with VV116 treatment. 60 patients received VV116 300mg twice daily for 5 days plus standard care, while 76 patients received only standard care. VV116 was generally safe, with only mild adverse events reported.
Standard of Care (SOC) for COVID-19 in the study country,
China, is average with moderate efficacy for approved treatments1.
|
time to viral-, 10.9% lower, relative time 0.89, p = 0.07, treatment mean 9.92 (±3.38) n=60, control mean 11.13 (±4.05) n=76, all patients.
|
|
time to viral-, 23.1% lower, relative time 0.77, p = 0.001, treatment mean 8.56 (±2.66) n=32, control mean 11.13 (±4.05) n=76, ≤5 days.
|
|
time to viral-, 3.0% higher, relative time 1.03, p = 0.71, treatment mean 11.46 (±3.6) n=28, control mean 11.13 (±4.05) n=76, >5 days.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Shen et al., 2 Jun 2022, prospective, China, peer-reviewed, mean age 33.9, 23 authors, study period 8 March, 2022 - 24 March, 2022, trial NCT05242042 (history).
Contact: zhangwenhong@fudan.edu.cn, fanxiaohong@shphc.org.cn.
An open, prospective cohort study of VV116 in Chinese participants infected with SARS-CoV-2 omicron variants
Emerging Microbes & Infections, doi:10.1080/22221751.2022.2078230
Omicron variant of SARS-CoV-2 has become the predominant variant worldwide. VV116 is an oral drug with robust anti-SARS-CoV-2 efficacy in preclinical studies. We conducted an open, prospective cohort study to evaluate its safety and effectiveness in Chinese participants infected with the omicron variant from March 8th, 2022 to March 24th, 2022. 136 hospitalized nonsevere patients confirmed with COVID-19 were enrolled including 60 patients who received VV116 (300 mg, BID×5 days) in the treatment group and 76 patients who didn't receive VV116 in the control group besides standard treatment. Viral load shedding time and adverse events were collected during the follow-up. There was no significant difference in baseline characteristics between the VV116 group and the control group, except for a higher symptom prevalence in the control group (P = 0.021). The median time from the first positive test to the first VV116 administration was 5 (range: 2-10) days. Participants who received VV116 within 5 days since the first positive test had a shorter viral shedding time than the control group (8.56 vs 11.13 days), and cox regression analysis showed adjusted HR of 2.37 [95%CI 1.50-3.75], P < 0.001. In symptomatic subgroup, VV116 group had a shorter viral shedding time than the control group (P = 0.016). A total of 9 adverse events with no serious adverse events were reported in the VV116 group, all of them were resolved without intervention. VV116 is a safe, effective oral antiviral drug, which shows a better performance within the early onset of omicron infection.
References
Bernal, Da Silva, Musungaie, Molnupiravir for oral treatment of COVID-19 in nonhospitalized patients, N Engl J Med
Cao, Wang, Jian, Omicron escapes the majority of existing SARS-CoV-2 neutralizing antibodies, Nature, doi:10.1038/s41586-021-04385-3
Christensen, Olsen, Long, Signals of significantly increased vaccine breakthrough, decreased hospitalization rates, and less severe disease in patients with Coronavirus disease 2019 caused by the omicron variant of severe acute Respiratory syndrome Coronavirus 2 in Houston, Texas, Am J Pathol
Dejnirattisai, Huo, Zhou, SARS-CoV-2 Omicron-B.1.1.529 leads to widespread escape from neutralizing antibody responses, Cell
Ekpanyapong, Bunchorntavakul, Reddy, COVID-19 and the liver: lessons learnt from the EAST and the WEST, A year later, J Viral Hepat
Hammond, Leister-Tebbe, Gardner, Oral nirmatrelvir for high-risk, Nonhospitalized adults with COVID-19, N Engl J Med
Mahase, COVID-19: Pfizer's paxlovid is 89% effective in patients at risk of serious illness, company reports, Br Med J
Qian, Wang, Zhang, Safety, tolerability, and pharmacokinetics of VV116, an oral nucleoside analog against SARS-CoV-2, in Chinese healthy subjects, Acta Pharmacol Sin, doi:10.1038/s41401-022-00895-6
Spinner, Gottlieb, Criner, Effect of Remdesivir vs standard care on Clinical status at 11 days in patients With moderate COVID-19: A randomized clinical trial, JAMA
Takashita, Kinoshita, Yamayoshi, Efficacy of antiviral agents against the SARS-CoV-2 Omicron Subvariant BA.2, N Engl J Med
Vangeel, Chiu, Jonghe, Molnupiravir and Nirmatrelvir remain active against SARS-CoV-2 Omicron and other variants of concern, Antiviral Res
Wang, Zhang, Du, Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial, The Lancet
Xie, Yin, Zhang, Design and development of an oral remdesivir derivative VV116 against SARS-CoV-2, Cell Res
Zhang, Shi, Wang, Liver injury in COVID-19: management and challenges, Lancet Gastroenterol Hepatol
Zhou, Yu, Du, Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study, The Lancet
DOI record:
{
"DOI": "10.1080/22221751.2022.2078230",
"ISSN": [
"2222-1751"
],
"URL": "http://dx.doi.org/10.1080/22221751.2022.2078230",
"alternative-id": [
"10.1080/22221751.2022.2078230"
],
"assertion": [
{
"label": "Peer Review Statement",
"name": "peerreview_statement",
"order": 1,
"value": "The publishing and review policy for this title is described in its Aims & Scope."
},
{
"URL": "http://www.tandfonline.com/action/journalInformation?show=aimsScope&journalCode=temi20",
"label": "Aim & Scope",
"name": "aims_and_scope_url",
"order": 2,
"value": "http://www.tandfonline.com/action/journalInformation?show=aimsScope&journalCode=temi20"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Received",
"name": "received",
"order": 0,
"value": "2022-04-26"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Accepted",
"name": "accepted",
"order": 1,
"value": "2022-05-11"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Published",
"name": "published",
"order": 2,
"value": "2022-06-02"
}
],
"author": [
{
"ORCID": "http://orcid.org/0000-0002-7604-5139",
"affiliation": [
{
"name": "Department of Infection and Immunity, Shanghai Public Health Clinical Centre, Fudan University, Shanghai, People’s Republic of China"
}
],
"authenticated-orcid": false,
"family": "Shen",
"given": "Yinzhong",
"sequence": "first"
},
{
"affiliation": [
{
"name": "Department of Infectious Diseases, National Medical Centre for Infectious Diseases, National Clinical Research Centre for Aging and Medicine, Shanghai Key Laboratory of Infectious Diseases and Biosafety Emergency Response, Huashan Hospital, Fudan University, Shanghai, People’s Republic of China"
}
],
"family": "Ai",
"given": "Jingwen",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of COVID-19, Shanghai Public Health Clinical Centre, Fudan University, Shanghai, People’s Republic of China"
}
],
"family": "Lin",
"given": "Na",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Infectious Diseases, National Medical Centre for Infectious Diseases, National Clinical Research Centre for Aging and Medicine, Shanghai Key Laboratory of Infectious Diseases and Biosafety Emergency Response, Huashan Hospital, Fudan University, Shanghai, People’s Republic of China"
}
],
"family": "Zhang",
"given": "Haocheng",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Infectious Diseases, National Medical Centre for Infectious Diseases, National Clinical Research Centre for Aging and Medicine, Shanghai Key Laboratory of Infectious Diseases and Biosafety Emergency Response, Huashan Hospital, Fudan University, Shanghai, People’s Republic of China"
}
],
"family": "Li",
"given": "Yang",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Infectious Diseases, National Medical Centre for Infectious Diseases, National Clinical Research Centre for Aging and Medicine, Shanghai Key Laboratory of Infectious Diseases and Biosafety Emergency Response, Huashan Hospital, Fudan University, Shanghai, People’s Republic of China"
}
],
"family": "Wang",
"given": "Hongyu",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Infectious Diseases, National Medical Centre for Infectious Diseases, National Clinical Research Centre for Aging and Medicine, Shanghai Key Laboratory of Infectious Diseases and Biosafety Emergency Response, Huashan Hospital, Fudan University, Shanghai, People’s Republic of China"
}
],
"family": "Wang",
"given": "Sen",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Lingang Laboratory, Shanghai, People’s Republic of China"
}
],
"family": "Wang",
"given": "Zhen",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Tuberculosis, Shanghai Public Health Clinical Centre, Fudan University, Shanghai, People’s Republic of China"
}
],
"family": "Li",
"given": "Tao",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Infectious Diseases, National Medical Centre for Infectious Diseases, National Clinical Research Centre for Aging and Medicine, Shanghai Key Laboratory of Infectious Diseases and Biosafety Emergency Response, Huashan Hospital, Fudan University, Shanghai, People’s Republic of China"
}
],
"family": "Sun",
"given": "Feng",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of COVID-19, Shanghai Public Health Clinical Centre, Fudan University, Shanghai, People’s Republic of China"
}
],
"family": "Fan",
"given": "Zhenyu",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of COVID-19, Shanghai Public Health Clinical Centre, Fudan University, Shanghai, People’s Republic of China"
}
],
"family": "Li",
"given": "Liqun",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of COVID-19, Shanghai Public Health Clinical Centre, Fudan University, Shanghai, People’s Republic of China"
}
],
"family": "Lu",
"given": "Yunfei",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of COVID-19, Shanghai Public Health Clinical Centre, Fudan University, Shanghai, People’s Republic of China"
}
],
"family": "Meng",
"given": "Xianmin",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of COVID-19, Shanghai Public Health Clinical Centre, Fudan University, Shanghai, People’s Republic of China"
}
],
"family": "Xiao",
"given": "Hong",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of COVID-19, Shanghai Public Health Clinical Centre, Fudan University, Shanghai, People’s Republic of China"
}
],
"family": "Hu",
"given": "Huiliang",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Infectious Diseases, Shanghai Public Health Clinical Centre, Fudan University, Shanghai, People’s Republic of China"
}
],
"family": "Ling",
"given": "Yun",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Respiratory Diseases, Shanghai Public Health Clinical Centre, Fudan University, Shanghai, People’s Republic of China"
}
],
"family": "Li",
"given": "Feng",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of COVID-19, Shanghai Public Health Clinical Centre, Fudan University, Shanghai, People’s Republic of China"
}
],
"family": "Li",
"given": "Hongdi",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of COVID-19, Shanghai Public Health Clinical Centre, Fudan University, Shanghai, People’s Republic of China"
}
],
"family": "Xi",
"given": "Chunmei",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of COVID-19, Shanghai Public Health Clinical Centre, Fudan University, Shanghai, People’s Republic of China"
}
],
"family": "Gu",
"given": "Liping",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Infectious Diseases, National Medical Centre for Infectious Diseases, National Clinical Research Centre for Aging and Medicine, Shanghai Key Laboratory of Infectious Diseases and Biosafety Emergency Response, Huashan Hospital, Fudan University, Shanghai, People’s Republic of China"
},
{
"name": "Department of Tuberculosis, Shanghai Public Health Clinical Centre, Fudan University, Shanghai, People’s Republic of China"
},
{
"name": "National Clinical Research Centre for Aging and Medicine, Huashan Hospital, Fudan University, Shanghai, People’s Republic of China"
},
{
"name": "Key Laboratory of Medical Molecular Virology (MOE/MOH), Shanghai Medical College, Fudan University, Shanghai, People’s Republic of China"
}
],
"family": "Zhang",
"given": "Wenhong",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Respiratory Diseases, Shanghai Public Health Clinical Centre, Fudan University, Shanghai, People’s Republic of China"
}
],
"family": "Fan",
"given": "Xiaohong",
"sequence": "additional"
}
],
"container-title": "Emerging Microbes & Infections",
"container-title-short": "Emerging Microbes & Infections",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"www.tandfonline.com"
]
},
"created": {
"date-parts": [
[
2022,
5,
17
]
],
"date-time": "2022-05-17T16:28:47Z",
"timestamp": 1652804927000
},
"deposited": {
"date-parts": [
[
2023,
10,
2
]
],
"date-time": "2023-10-02T17:59:00Z",
"timestamp": 1696269540000
},
"funder": [
{
"DOI": "10.13039/501100003399",
"award": [
"22YJ1900102",
"22YJ1900100"
],
"doi-asserted-by": "publisher",
"name": "Shanghai Science and Technology Committee"
},
{
"award": [
"shslczdzk01102"
],
"name": "clinical key specialty project on Shanghai infectious diseases"
},
{
"DOI": "10.13039/501100003399",
"award": [
"21NL2600100",
"20dz2260100",
"20dz2210400"
],
"doi-asserted-by": "publisher",
"name": "Shanghai Municipal Science and Technology Major Project, Shanghai Science and Technology Committee"
},
{
"DOI": "10.13039/501100010032",
"award": [
"GWV-10.1-XK01",
"GWV-3.1",
"GWV-2"
],
"doi-asserted-by": "publisher",
"name": "Shanghai Municipal Health Commission"
},
{
"award": [
"LG-YJ-202204-02"
],
"name": "Lingang Laboratory"
}
],
"indexed": {
"date-parts": [
[
2024,
1,
2
]
],
"date-time": "2024-01-02T20:43:51Z",
"timestamp": 1704228231081
},
"is-referenced-by-count": 40,
"issue": "1",
"issued": {
"date-parts": [
[
2022,
6,
2
]
]
},
"journal-issue": {
"issue": "1",
"published-print": {
"date-parts": [
[
2022,
12,
31
]
]
}
},
"language": "en",
"license": [
{
"URL": "http://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
6,
2
]
],
"date-time": "2022-06-02T00:00:00Z",
"timestamp": 1654128000000
}
}
],
"link": [
{
"URL": "https://www.tandfonline.com/doi/pdf/10.1080/22221751.2022.2078230",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "301",
"original-title": [],
"page": "1518-1523",
"prefix": "10.1080",
"published": {
"date-parts": [
[
2022,
6,
2
]
]
},
"published-online": {
"date-parts": [
[
2022,
6,
2
]
]
},
"published-print": {
"date-parts": [
[
2022,
12,
31
]
]
},
"publisher": "Informa UK Limited",
"reference": [
{
"DOI": "10.1016/j.ajpath.2022.01.007",
"doi-asserted-by": "publisher",
"key": "e_1_3_3_2_1"
},
{
"DOI": "10.1016/j.cell.2021.12.046",
"doi-asserted-by": "publisher",
"key": "e_1_3_3_3_1"
},
{
"DOI": "10.1016/j.antiviral.2022.105252",
"doi-asserted-by": "publisher",
"key": "e_1_3_3_4_1"
},
{
"DOI": "10.1056/NEJMc2201933",
"doi-asserted-by": "publisher",
"key": "e_1_3_3_5_1"
},
{
"DOI": "10.1038/s41422-021-00570-1",
"doi-asserted-by": "publisher",
"key": "e_1_3_3_6_1"
},
{
"DOI": "10.1038/s41401-022-00895-6",
"doi-asserted-by": "publisher",
"key": "e_1_3_3_7_1"
},
{
"key": "e_1_3_3_8_1",
"unstructured": "COVID-19 map in China. https://voice.baidu.com/act/newpneumonia/newpneumonia/?from = osari_aladin_banner&city = %E4%B8%8A%E6%B5%B7-%E4%B8%8A%E6%B5%B7 (Accessed 23 Apr 2022)."
},
{
"DOI": "10.1016/S0140-6736(20)30566-3",
"doi-asserted-by": "publisher",
"key": "e_1_3_3_9_1"
},
{
"DOI": "10.1016/S0140-6736(20)31022-9",
"doi-asserted-by": "publisher",
"key": "e_1_3_3_10_1"
},
{
"DOI": "10.1001/jama.2020.16349",
"doi-asserted-by": "publisher",
"key": "e_1_3_3_11_1"
},
{
"key": "e_1_3_3_12_1",
"unstructured": "Commissioner O of the FDA Approves First Treatment for COVID-19. FDA. https://www.fda.gov/news-events/press-announcements/fda-approves-first-treatment-covid-19 (accessed 23 Apr2022); 2020."
},
{
"DOI": "10.1136/bmj.n2713",
"doi-asserted-by": "publisher",
"key": "e_1_3_3_13_1"
},
{
"DOI": "10.1056/NEJMoa2116044",
"doi-asserted-by": "publisher",
"key": "e_1_3_3_14_1"
},
{
"DOI": "10.1038/s41586-021-04385-3",
"doi-asserted-by": "publisher",
"key": "e_1_3_3_15_1"
},
{
"DOI": "10.1056/NEJMoa2118542",
"doi-asserted-by": "publisher",
"key": "e_1_3_3_16_1"
},
{
"DOI": "10.1016/S2468-1253(20)30057-1",
"doi-asserted-by": "publisher",
"key": "e_1_3_3_17_1"
},
{
"DOI": "10.1111/jvh.13590",
"doi-asserted-by": "publisher",
"key": "e_1_3_3_18_1"
}
],
"reference-count": 17,
"references-count": 17,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.tandfonline.com/doi/full/10.1080/22221751.2022.2078230"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Virology",
"Infectious Diseases",
"Drug Discovery",
"General Medicine",
"Immunology",
"Microbiology",
"Parasitology",
"Epidemiology"
],
"subtitle": [],
"title": "An open, prospective cohort study of VV116 in Chinese participants infected with SARS-CoV-2 omicron variants",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1080/tandf_crossmark_01",
"volume": "11"
}