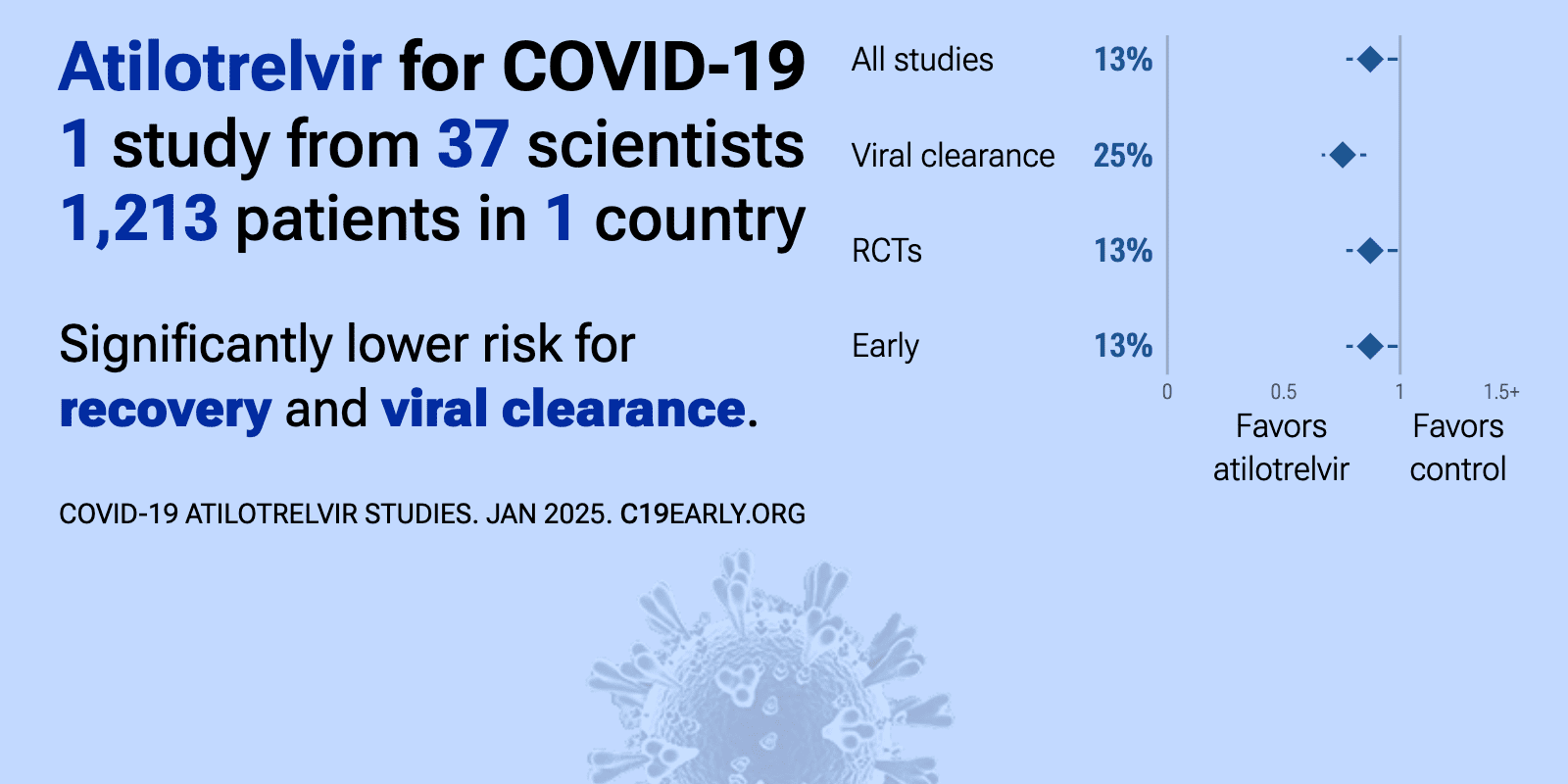
Atilotrelvir was adopted
in 1 country.
May 31 2024 |
et al., eClinicalMedicine, doi:10.1016/j.eclinm.2024.102582 | Efficacy and safety of GST-HG171 in adult patients with mild to moderate COVID-19: a randomised, double-blind, placebo-controlled phase 2/3 trial |
| 13% faster recovery (p=0.03) and 25% faster viral clearance (p<0.0001). RCT 1,213 outpatients with mild-to-moderate COVID-19 in China showing a shorter time to sustained recovery and faster viral clearance with GST-HG171 (atilotrelvir) plus ritonavir. | ||