Efficacy and safety of GST-HG171 in adult patients with mild to moderate COVID-19: a randomised, double-blind, placebo-controlled phase 2/3 trial
et al., eClinicalMedicine, doi:10.1016/j.eclinm.2024.102582, NCT05656443, May 2024
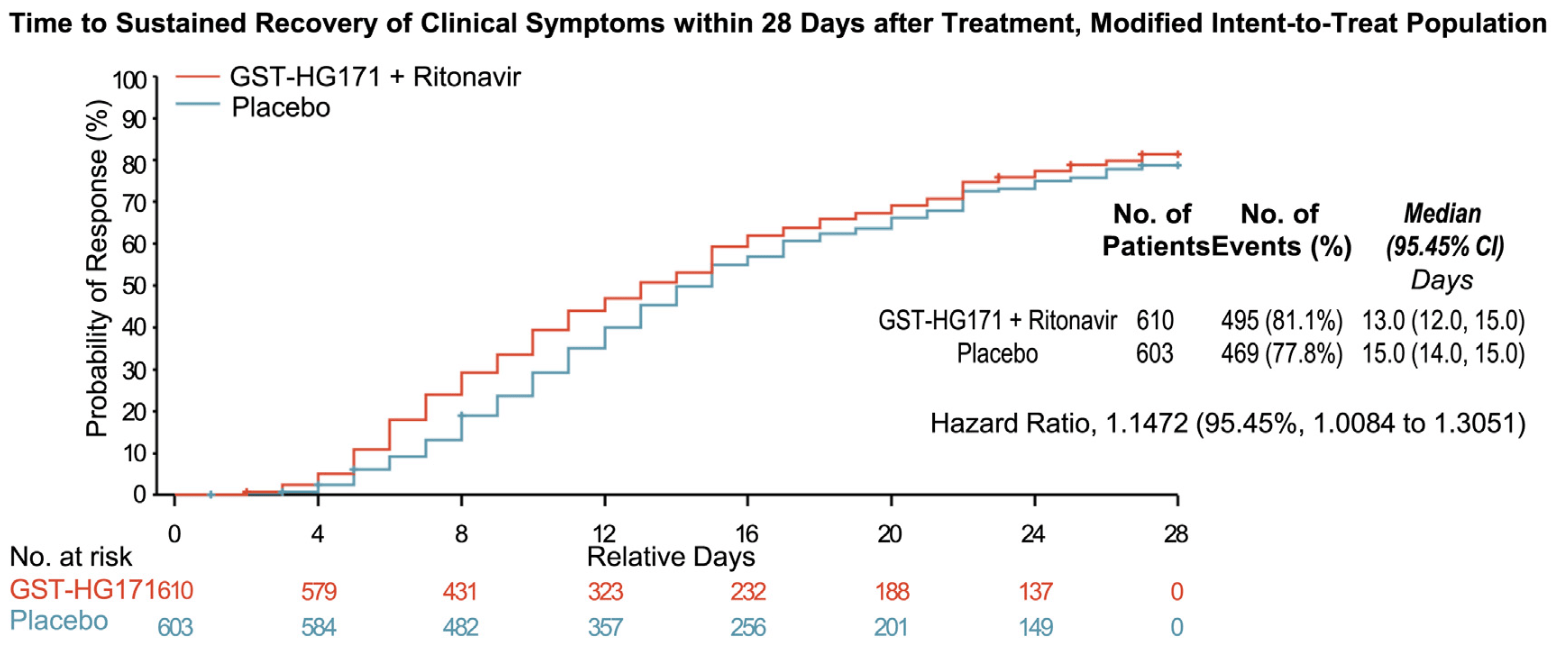
RCT 1,213 outpatients with mild-to-moderate COVID-19 in China showing a shorter time to sustained recovery and faster viral clearance with GST-HG171 (atilotrelvir) plus ritonavir.
Standard of Care (SOC) for COVID-19 in the study country,
China, is average with moderate efficacy for approved treatments1.
|
time to sustained recovery, 12.8% lower, HR 0.87, p = 0.03, treatment 610, control 603, inverted to make HR<1 favor treatment.
|
|
time to negative conversion, 24.7% lower, HR 0.75, p < 0.001, treatment 610, control 607, inverted to make HR<1 favor treatment.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Lu et al., 31 May 2024, Double Blind Randomized Controlled Trial, placebo-controlled, China, peer-reviewed, mean age 34.5, 37 authors, study period 19 December, 2022 - 4 May, 2023, trial NCT05656443 (history).
Contact: nanshan@vip.163.com, luhongzhou@fudan.edu.cn, george.zhang@akeylink.cn, chenxc998@fjmu.edu.cn, zhan071119@163.com, linl6@163.com, tangyanan@cosunter.com, lin_fenghn@126.com, hnldzfy@126.com, 553039465@qq.com, yi_ming@126.com, kaiyu@jlu.edu.cn, 413699442@qq.com, 490458234@qq.com, hly0311@sina.com, hp_cq@163.com, yhlin_xm@163.com, zxbqz@163.com, connie.chen@tigermedgrp.com, yanwenhao@akeylink.cn, yanxiuping@akeylink.cn, mu@tigermedgrp.com, ella.lin@tigermedgrp.com, tuxinyu1997@gmail.com, tanhongshan@akeylink.cn, huzhiqiang@cosunter.com, lihongming@cosunter.com, lgp@cosunter.com, jeffyah@163.com, chen_xinwen@gzlab.ac.cn, fswang302@163.com.
Efficacy and safety of GST-HG171 in adult patients with mild to moderate COVID-19: a randomised, double-blind, placebo-controlled phase 2/3 trial
eClinicalMedicine, doi:10.1016/j.eclinm.2024.102582
Background GST-HG171 is a potent, broad-spectrum, orally bioavailable small-molecule 3C like protease inhibitor that has demonstrated greater potency and efficacy compared to Nirmatrelvir in pre-clinical studies. We aimed to evaluate the efficacy and safety of orally administered GST-HG171 plus Ritonavir in patients with coronavirus disease 2019 (COVID-19) infected with emerging XBB and non-XBB variants. Methods This randomised, double-blind, placebo-controlled phase 2/3 trial was conducted in 47 sites in China among adult patients with mild-to-moderate COVID-19 with symptoms onset ≤72 h. Eligible patients were randomised 1:1 to receive GST-HG171 (150 mg) plus Ritonavir (100 mg) or corresponding placebo tablets twice daily for 5 days, with stratification factors including the risk level of disease progression and vaccination status. The primary efficacy endpoint was time to sustained recovery of clinical symptoms within 28 days, defined as a score of 0 for 11
Contributors NZ, HongzL, GZ, JM, YZ, XiaC, ZY, XinC, GW, and XiaoC conceived and designed the study. NZ and HongzL are co-principal investigators, and FL, LL, FZ, YL, YZ, KZ, WenfY, RS, LH, PH, YL, XZ, and FW are investigators. YT, TZ, WenhY, XY, HT, LM, FH, ZH, and ZY executed the study, with supervision from GZ, JM, YZ, ZY, ZheL, HongmL, and GL. ZW, ZhuL, and HF contributed to statistical analysis. GZ, XiaC, and XT drafted the manuscript, with critical revision by NZ, HongzL, JM, YZ, TZ, XY, ZhuL, GW, XiaoC, XinC, and FW. NZ, HongzL, and GZ have directly accessed and verified the underlying data reported in the manuscript. All authors had full access to all the data in the study, and approved of this manuscript to be submitted for publication.
Data sharing statement After approval from the Human Genetic Resources Administration of China, this trial data can be shared with qualifying researchers who submit a proposal with a valuable research question. A contract should be signed.
Declaration of interests
References
Bernal, Da Silva, Musungaie, Molnupiravir for oral treatment of covid-19 in nonhospitalized patients, N Engl J Med
Brown, Brien, Snow, A prospective study of key correlates for household transmission of severe acute respiratory syndrome coronavirus 2, Open Forum Infect Dis
Butler, Hobbs, Gbinigie, Molnupiravir plus usual care versus usual care alone as early treatment for adults with COVID-19 at increased risk of adverse outcomes (PANORAMIC): an open-label, platform-adaptive randomised controlled trial, Lancet
Cao, Gao, Bao, VV116 versus nirmatrelvir-ritonavir for oral treatment of covid-19, N Engl J Med
Cao, Wang, Lu, Oral Simnotrelvir for adult patients with mild-to-moderate covid-19, N Engl J Med
Fajnzylber, Regan, Coxen, SARS-CoV-2 viral load is associated with increased disease severity and mortality, Nat Commun
Fan, Dai, Ling, Oral VV116 versus placebo in patients with mild-to-moderate COVID-19 in China: a multicentre, doubleblind, phase 3, randomised controlled study, Lancet Infect Dis
Gong, Zhu, Li, An online coronavirus analysis platform from the National Genomics Data Center, Zool Res
Gottlieb, Vaca, Paredes, Early Remdesivir to prevent progression to severe covid-19 in outpatients, N Engl J Med
Hachmann, Miller, Collier, Neutralization escape by SARS-CoV-2 omicron subvariants BA, BA
Hammond, Leister-Tebbe, Gardner, Oral Nirmatrelvir for high-risk, nonhospitalized adults with covid-19, N Engl J Med
Lan, Demets, Discrete sequential boundaries for clinical trials, Biometrika
Maimeri, Marmiere, Losiggio, Interventions reducing mortality in COVID-19 patients: a systematic review of randomized evidence, Minerva Med
Mukae, Yotsuyanagi, Ohmagari, Efficacy and safety of Ensitrelvir in patients with mild-to-moderate coronavirus disease 2019: the phase 2b part of a randomized, placebo-controlled, phase 2/3 study, Clin Infect Dis
Nyberg, Ferguson, Nash, Comparative analysis of the risks of hospitalisation and death associated with SARS-CoV-2 omicron (B.1.1.529) and delta (B.1.617.2) variants in England: a cohort study, Lancet
O'brien, Fleming, A multiple testing procedure for clinical trials, Biometrics
Reis, Santos Moreira-Silva, Silva, Effect of early treatment with fluvoxamine on risk of emergency care and hospitalisation among patients with COVID-19: the TOGETHER randomised, platform clinical trial [published correction appears in, Lancet Glob Health
Song, Ma, Zou, The global landscape of SARS-CoV-2 genomes, variants, and haplotypes in 2019nCoVR, Dev Reprod Biol
Wang, Guo, Iketani, Antibody evasion by SARS-CoV-2 Omicron subvariants BA, BA.4 and BA.5
Wolter, Jassat, Walaza, Early assessment of the clinical severity of the SARS-CoV-2 omicron variant in South Africa: a data linkage study, Lancet
Yotsuyanagi, Ohmagari, Doi, Efficacy and safety of 5day oral Ensitrelvir for patients with mild to moderate COVID-19: the SCORPIO-SR randomized clinical trial, JAMA Netw Open
Yu, Bafadhel, Dorward, Inhaled budesonide for COVID-19 in people at high risk of complications in the community in the UK (PRINCIPLE): a randomised, controlled, open-label, adaptive platform trial, Lancet
Yu, Yang, Tang, Coronavirus GenBrowser for monitoring the transmission and evolution of SARS-CoV-2, Brief Bioinform
Zhan, Lin, Liang, Leritrelvir for the treatment of mild or moderate COVID-19 without co-administered ritonavir: a multicentre randomised, double-blind, placebo-controlled phase 3 trial, eClinicalMedicine
Zhang, Mao, He, Discovery of GST-HG171, A potent and selective oral 3CL protease inhibitor for the treatment of COVID-19, SM J Infect Dis
Zhang, Zhou, Chen, Phase I study, and dosing regimen selection for a pivotal COVID-19 trial of GST-HG171
Zhao, Song, Chen, The 2019 novel coronavirus resource, Yi Chuan
DOI record:
{
"DOI": "10.1016/j.eclinm.2024.102582",
"ISSN": [
"2589-5370"
],
"URL": "http://dx.doi.org/10.1016/j.eclinm.2024.102582",
"alternative-id": [
"S2589537024001615"
],
"article-number": "102582",
"assertion": [
{
"label": "This article is maintained by",
"name": "publisher",
"value": "Elsevier"
},
{
"label": "Article Title",
"name": "articletitle",
"value": "Efficacy and safety of GST-HG171 in adult patients with mild to moderate COVID-19: a randomised, double-blind, placebo-controlled phase 2/3 trial"
},
{
"label": "Journal Title",
"name": "journaltitle",
"value": "eClinicalMedicine"
},
{
"label": "CrossRef DOI link to publisher maintained version",
"name": "articlelink",
"value": "https://doi.org/10.1016/j.eclinm.2024.102582"
},
{
"label": "Content Type",
"name": "content_type",
"value": "article"
},
{
"label": "Copyright",
"name": "copyright",
"value": "© 2024 The Author(s). Published by Elsevier Ltd."
}
],
"author": [
{
"ORCID": "http://orcid.org/0000-0002-8308-5534",
"affiliation": [],
"authenticated-orcid": false,
"family": "Lu",
"given": "Hongzhou",
"sequence": "first"
},
{
"ORCID": "http://orcid.org/0000-0001-5437-6028",
"affiliation": [],
"authenticated-orcid": false,
"family": "Zhang",
"given": "George",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mao",
"given": "John",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Chen",
"given": "Xiaochun",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Zhan",
"given": "Yangqing",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lin",
"given": "Ling",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Zhang",
"given": "Tianxiang",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Tang",
"given": "Yanan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lin",
"given": "Feng",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Zhu",
"given": "Feiyue",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lin",
"given": "Yuanlong",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-5635-3006",
"affiliation": [],
"authenticated-orcid": false,
"family": "Zeng",
"given": "Yiming",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Zhang",
"given": "Kaiyu",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Yuan",
"given": "Wenfang",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Liang",
"given": "Zhenyu",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Sun",
"given": "Ruilin",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Huo",
"given": "Liya",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hu",
"given": "Peng",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lin",
"given": "Yihua",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Zhuang",
"given": "Xibin",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Wei",
"given": "Zhaohui",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Chen",
"given": "Xia",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Yan",
"given": "Wenhao",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Yan",
"given": "Xiuping",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mu",
"given": "Lisa",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lin",
"given": "Zhuhua",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Tu",
"given": "Xinyu",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Tan",
"given": "Hongshan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Huang",
"given": "Fuhu",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hu",
"given": "Zhiqiang",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Li",
"given": "Hongming",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Li",
"given": "Guoping",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Fu",
"given": "Haijun",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Yang",
"given": "Zifeng",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Chen",
"given": "Xinwen",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Wang",
"given": "Fu-Sheng",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Zhong",
"given": "Nanshan",
"sequence": "additional"
}
],
"container-title": "eClinicalMedicine",
"container-title-short": "eClinicalMedicine",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"thelancet.com",
"elsevier.com",
"sciencedirect.com"
]
},
"created": {
"date-parts": [
[
2024,
4,
10
]
],
"date-time": "2024-04-10T09:53:30Z",
"timestamp": 1712742810000
},
"deposited": {
"date-parts": [
[
2024,
5,
18
]
],
"date-time": "2024-05-18T04:57:41Z",
"timestamp": 1716008261000
},
"indexed": {
"date-parts": [
[
2024,
5,
19
]
],
"date-time": "2024-05-19T01:03:14Z",
"timestamp": 1716080594644
},
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2024,
5
]
]
},
"language": "en",
"license": [
{
"URL": "https://www.elsevier.com/tdm/userlicense/1.0/",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2024,
5,
1
]
],
"date-time": "2024-05-01T00:00:00Z",
"timestamp": 1714521600000
}
},
{
"URL": "https://www.elsevier.com/legal/tdmrep-license",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2024,
5,
1
]
],
"date-time": "2024-05-01T00:00:00Z",
"timestamp": 1714521600000
}
},
{
"URL": "http://creativecommons.org/licenses/by-nc-nd/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2024,
3,
19
]
],
"date-time": "2024-03-19T00:00:00Z",
"timestamp": 1710806400000
}
}
],
"link": [
{
"URL": "https://api.elsevier.com/content/article/PII:S2589537024001615?httpAccept=text/xml",
"content-type": "text/xml",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://api.elsevier.com/content/article/PII:S2589537024001615?httpAccept=text/plain",
"content-type": "text/plain",
"content-version": "vor",
"intended-application": "text-mining"
}
],
"member": "78",
"original-title": [],
"page": "102582",
"prefix": "10.1016",
"published": {
"date-parts": [
[
2024,
5
]
]
},
"published-print": {
"date-parts": [
[
2024,
5
]
]
},
"publisher": "Elsevier BV",
"reference": [
{
"DOI": "10.1056/NEJMc2206576",
"article-title": "Neutralization escape by SARS-CoV-2 omicron subvariants BA.2.12.1, BA.4, and BA.5",
"author": "Hachmann",
"doi-asserted-by": "crossref",
"first-page": "86",
"issue": "1",
"journal-title": "N Engl J Med",
"key": "10.1016/j.eclinm.2024.102582_bib1",
"volume": "387",
"year": "2022"
},
{
"DOI": "10.1038/s41586-022-05053-w",
"article-title": "Antibody evasion by SARS-CoV-2 Omicron subvariants BA.2.12.1, BA.4 and BA.5",
"author": "Wang",
"doi-asserted-by": "crossref",
"first-page": "603",
"issue": "7923",
"journal-title": "Nature",
"key": "10.1016/j.eclinm.2024.102582_bib2",
"volume": "608",
"year": "2022"
},
{
"article-title": "The global landscape of SARS-CoV-2 genomes, variants, and haplotypes in 2019nCoVR",
"author": "Song",
"first-page": "749",
"issue": "6",
"journal-title": "Dev Reprod Biol",
"key": "10.1016/j.eclinm.2024.102582_bib3",
"volume": "18",
"year": "2020"
},
{
"article-title": "The 2019 novel coronavirus resource",
"author": "Zhao",
"first-page": "212",
"issue": "2",
"journal-title": "Yi Chuan",
"key": "10.1016/j.eclinm.2024.102582_bib4",
"volume": "42",
"year": "2020"
},
{
"DOI": "10.24272/j.issn.2095-8137.2020.065",
"article-title": "An online coronavirus analysis platform from the National Genomics Data Center",
"author": "Gong",
"doi-asserted-by": "crossref",
"first-page": "705",
"issue": "6",
"journal-title": "Zool Res",
"key": "10.1016/j.eclinm.2024.102582_bib5",
"volume": "41",
"year": "2020"
},
{
"DOI": "10.1093/bib/bbab583",
"article-title": "Coronavirus GenBrowser for monitoring the transmission and evolution of SARS-CoV-2",
"author": "Yu",
"doi-asserted-by": "crossref",
"first-page": "bbab583",
"issue": "2",
"journal-title": "Brief Bioinform",
"key": "10.1016/j.eclinm.2024.102582_bib6",
"volume": "23",
"year": "2022"
},
{
"key": "10.1016/j.eclinm.2024.102582_bib7",
"series-title": "Report on epidemic of SARS-CoV-2 in China",
"year": "2023"
},
{
"DOI": "10.1016/S0140-6736(22)00462-7",
"article-title": "Comparative analysis of the risks of hospitalisation and death associated with SARS-CoV-2 omicron (B.1.1.529) and delta (B.1.617.2) variants in England: a cohort study",
"author": "Nyberg",
"doi-asserted-by": "crossref",
"first-page": "1303",
"issue": "10332",
"journal-title": "Lancet",
"key": "10.1016/j.eclinm.2024.102582_bib8",
"volume": "399",
"year": "2022"
},
{
"DOI": "10.1016/S0140-6736(22)00017-4",
"article-title": "Early assessment of the clinical severity of the SARS-CoV-2 omicron variant in South Africa: a data linkage study",
"author": "Wolter",
"doi-asserted-by": "crossref",
"first-page": "437",
"issue": "10323",
"journal-title": "Lancet",
"key": "10.1016/j.eclinm.2024.102582_bib9",
"volume": "399",
"year": "2022"
},
{
"DOI": "10.1056/NEJMoa2116044",
"article-title": "Molnupiravir for oral treatment of covid-19 in nonhospitalized patients",
"author": "Jayk Bernal",
"doi-asserted-by": "crossref",
"first-page": "509",
"issue": "6",
"journal-title": "N Engl J Med",
"key": "10.1016/j.eclinm.2024.102582_bib10",
"volume": "386",
"year": "2022"
},
{
"DOI": "10.1056/NEJMoa2118542",
"article-title": "Oral Nirmatrelvir for high-risk, nonhospitalized adults with covid-19",
"author": "Hammond",
"doi-asserted-by": "crossref",
"first-page": "1397",
"issue": "15",
"journal-title": "N Engl J Med",
"key": "10.1016/j.eclinm.2024.102582_bib11",
"volume": "386",
"year": "2022"
},
{
"DOI": "10.1056/NEJMoa2208822",
"article-title": "VV116 versus nirmatrelvir-ritonavir for oral treatment of covid-19",
"author": "Cao",
"doi-asserted-by": "crossref",
"first-page": "406",
"issue": "5",
"journal-title": "N Engl J Med",
"key": "10.1016/j.eclinm.2024.102582_bib12",
"volume": "388",
"year": "2023"
},
{
"DOI": "10.1093/cid/ciac933",
"article-title": "Efficacy and safety of Ensitrelvir in patients with mild-to-moderate coronavirus disease 2019: the phase 2b part of a randomized, placebo-controlled, phase 2/3 study",
"author": "Mukae",
"doi-asserted-by": "crossref",
"first-page": "1403",
"issue": "8",
"journal-title": "Clin Infect Dis",
"key": "10.1016/j.eclinm.2024.102582_bib13",
"volume": "76",
"year": "2023"
},
{
"article-title": "Interventions reducing mortality in COVID-19 patients: a systematic review of randomized evidence",
"author": "Maimeri",
"first-page": "61",
"journal-title": "Minerva Med",
"key": "10.1016/j.eclinm.2024.102582_bib14",
"volume": "115",
"year": "2023"
},
{
"DOI": "10.1016/S2214-109X(21)00448-4",
"author": "Reis",
"doi-asserted-by": "crossref",
"first-page": "e42",
"issue": "1",
"journal-title": "Lancet Glob Health",
"key": "10.1016/j.eclinm.2024.102582_bib15",
"volume": "10",
"year": "2022"
},
{
"DOI": "10.1016/S0140-6736(22)02597-1",
"article-title": "Molnupiravir plus usual care versus usual care alone as early treatment for adults with COVID-19 at increased risk of adverse outcomes (PANORAMIC): an open-label, platform-adaptive randomised controlled trial",
"author": "Butler",
"doi-asserted-by": "crossref",
"first-page": "281",
"issue": "10373",
"journal-title": "Lancet",
"key": "10.1016/j.eclinm.2024.102582_bib17",
"volume": "401",
"year": "2023"
},
{
"DOI": "10.1016/S0140-6736(21)01744-X",
"article-title": "Inhaled budesonide for COVID-19 in people at high risk of complications in the community in the UK (PRINCIPLE): a randomised, controlled, open-label, adaptive platform trial [published correction appears in Lancet. 2021 Aug 18]",
"author": "Yu",
"doi-asserted-by": "crossref",
"first-page": "843",
"issue": "10303",
"journal-title": "Lancet",
"key": "10.1016/j.eclinm.2024.102582_bib18",
"volume": "398",
"year": "2021"
},
{
"DOI": "10.1001/jamanetworkopen.2023.54991",
"article-title": "Efficacy and safety of 5-day oral Ensitrelvir for patients with mild to moderate COVID-19: the SCORPIO-SR randomized clinical trial",
"author": "Yotsuyanagi",
"doi-asserted-by": "crossref",
"issue": "2",
"journal-title": "JAMA Netw Open",
"key": "10.1016/j.eclinm.2024.102582_bib19",
"volume": "7",
"year": "2024"
},
{
"DOI": "10.1016/S1473-3099(23)00577-7",
"article-title": "Oral VV116 versus placebo in patients with mild-to-moderate COVID-19 in China: a multicentre, double-blind, phase 3, randomised controlled study",
"author": "Fan",
"doi-asserted-by": "crossref",
"first-page": "129",
"journal-title": "Lancet Infect Dis",
"key": "10.1016/j.eclinm.2024.102582_bib20",
"volume": "24",
"year": "2023"
},
{
"DOI": "10.1056/NEJMoa2301425",
"article-title": "Oral Simnotrelvir for adult patients with mild-to-moderate covid-19",
"author": "Cao",
"doi-asserted-by": "crossref",
"first-page": "230",
"issue": "3",
"journal-title": "N Engl J Med",
"key": "10.1016/j.eclinm.2024.102582_bib21",
"volume": "390",
"year": "2024"
},
{
"article-title": "Leritrelvir for the treatment of mild or moderate COVID-19 without co-administered ritonavir: a multicentre randomised, double-blind, placebo-controlled phase 3 trial",
"author": "Zhan",
"journal-title": "eClinicalMedicine",
"key": "10.1016/j.eclinm.2024.102582_bib22",
"volume": "67",
"year": "2023"
},
{
"article-title": "Discovery of GST-HG171, A potent and selective oral 3CL protease inhibitor for the treatment of COVID-19",
"author": "Zhang",
"first-page": "9",
"journal-title": "SM J Infect Dis",
"key": "10.1016/j.eclinm.2024.102582_bib23",
"volume": "6",
"year": "2023"
},
{
"DOI": "10.1128/aac.01115-23",
"article-title": "Phase I study, and dosing regimen selection for a pivotal COVID-19 trial of GST-HG171",
"author": "Zhang",
"doi-asserted-by": "crossref",
"first-page": "e0111523",
"journal-title": "Antimicrob Agents Chemother",
"key": "10.1016/j.eclinm.2024.102582_bib24",
"volume": "68",
"year": "2023"
},
{
"key": "10.1016/j.eclinm.2024.102582_bib25",
"series-title": "Diagnosis and treatment protocol for COVID-19 (trial version 10) issued by the national health commission of the People's Republic of China",
"year": "2023"
},
{
"DOI": "10.2307/2336502",
"article-title": "Discrete sequential boundaries for clinical trials",
"author": "Lan",
"doi-asserted-by": "crossref",
"first-page": "659",
"journal-title": "Biometrika",
"key": "10.1016/j.eclinm.2024.102582_bib26",
"volume": "70",
"year": "1983"
},
{
"DOI": "10.2307/2530245",
"article-title": "A multiple testing procedure for clinical trials",
"author": "O'Brien",
"doi-asserted-by": "crossref",
"first-page": "549",
"journal-title": "Biometrics",
"key": "10.1016/j.eclinm.2024.102582_bib27",
"volume": "35",
"year": "1979"
},
{
"DOI": "10.1056/NEJMoa2116846",
"article-title": "Early Remdesivir to prevent progression to severe covid-19 in outpatients",
"author": "Gottlieb",
"doi-asserted-by": "crossref",
"first-page": "305",
"issue": "4",
"journal-title": "N Engl J Med",
"key": "10.1016/j.eclinm.2024.102582_bib28",
"volume": "386",
"year": "2022"
},
{
"DOI": "10.1038/s41467-020-19057-5",
"article-title": "SARS-CoV-2 viral load is associated with increased disease severity and mortality",
"author": "Fajnzylber",
"doi-asserted-by": "crossref",
"first-page": "5493",
"issue": "1",
"journal-title": "Nat Commun",
"key": "10.1016/j.eclinm.2024.102582_bib29",
"volume": "11",
"year": "2020"
},
{
"DOI": "10.1093/ofid/ofad271",
"article-title": "A prospective study of key correlates for household transmission of severe acute respiratory syndrome coronavirus 2",
"author": "Brown",
"doi-asserted-by": "crossref",
"issue": "7",
"journal-title": "Open Forum Infect Dis",
"key": "10.1016/j.eclinm.2024.102582_bib30",
"volume": "10",
"year": "2023"
}
],
"reference-count": 29,
"references-count": 29,
"relation": {},
"resource": {
"primary": {
"URL": "https://linkinghub.elsevier.com/retrieve/pii/S2589537024001615"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Efficacy and safety of GST-HG171 in adult patients with mild to moderate COVID-19: a randomised, double-blind, placebo-controlled phase 2/3 trial",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1016/elsevier_cm_policy",
"volume": "71"
}