Nov 2 2024 |
et al., Clinical Infectious Diseases, doi:10.1093/cid/ciae529 | Virologic Response and Safety of Ibuzatrelvir, a Novel SARS-CoV-2 Antiviral, in Adults With COVID-19 |
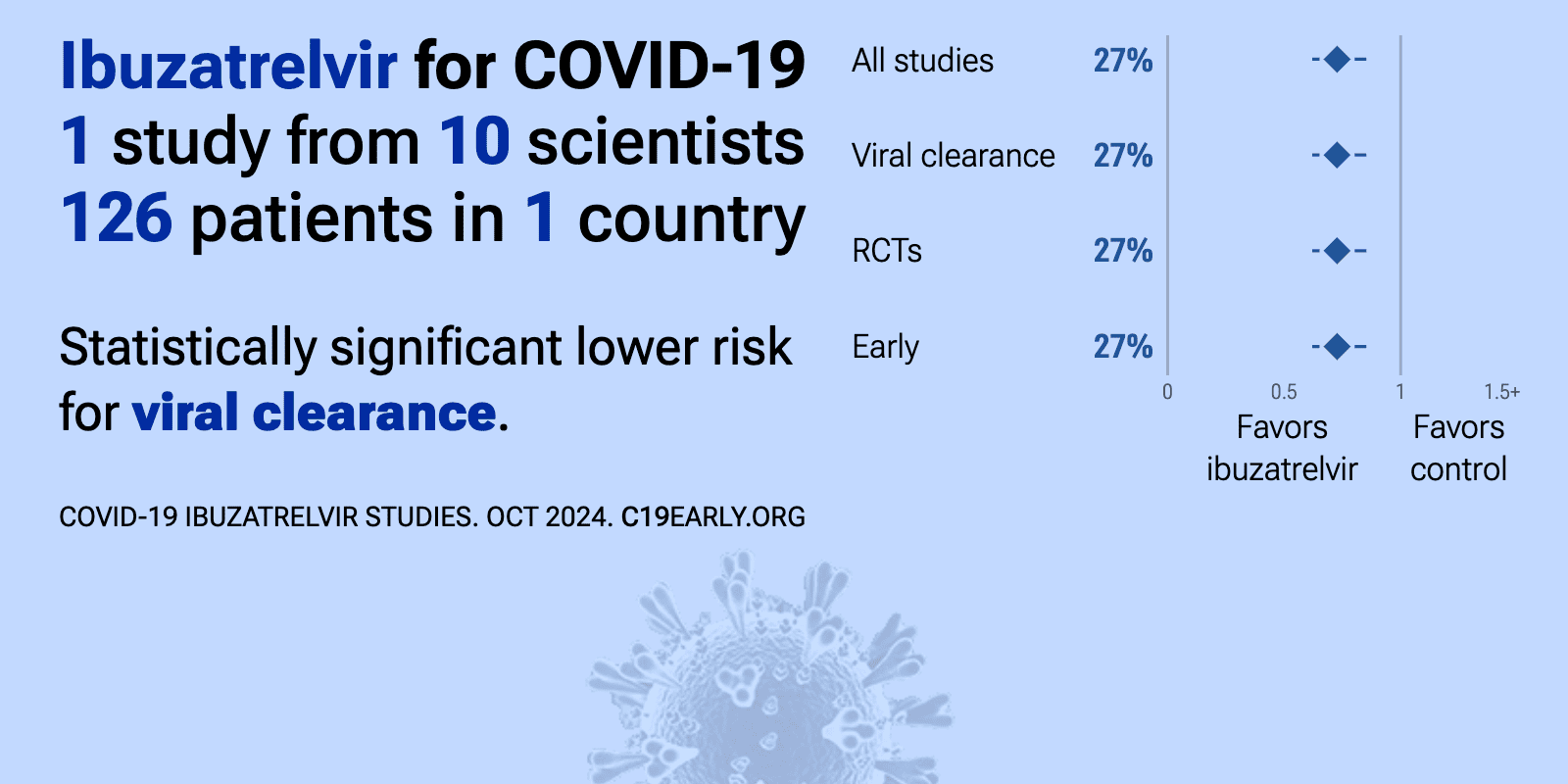
| 27% improved viral clearance (p=0.0001). RCT 240 outpatients showing significant viral load reduction with ibuzatrelvir (an oral SARS-CoV-2 Mpro inhibitor) compared to placebo. The study enrolled non-hospitalized adults aged 18-65 with symptomatic COVID-19 (≤5 days) and positive.. | ||
Jul 5 2024 |
et al., Journal of Medicinal Chemistry, doi:10.1021/acs.jmedchem.4c01342 | Fixing the Achilles Heel of Pfizer’s Paxlovid for COVID-19 Treatment |
| Perspective article discussing the development of ibuzatrelvir (PF-07817883) as a SARS-CoV-2 main protease (Mpro) inhibitor which addresses the metabolic instability of nirmatrelvir and does not require co-administration with ritonavir, t.. | ||