Virologic Response and Safety of Ibuzatrelvir, a Novel SARS-CoV-2 Antiviral, in Adults With COVID-19
et al., Clinical Infectious Diseases, doi:10.1093/cid/ciae529, NCT05799495, Nov 2024
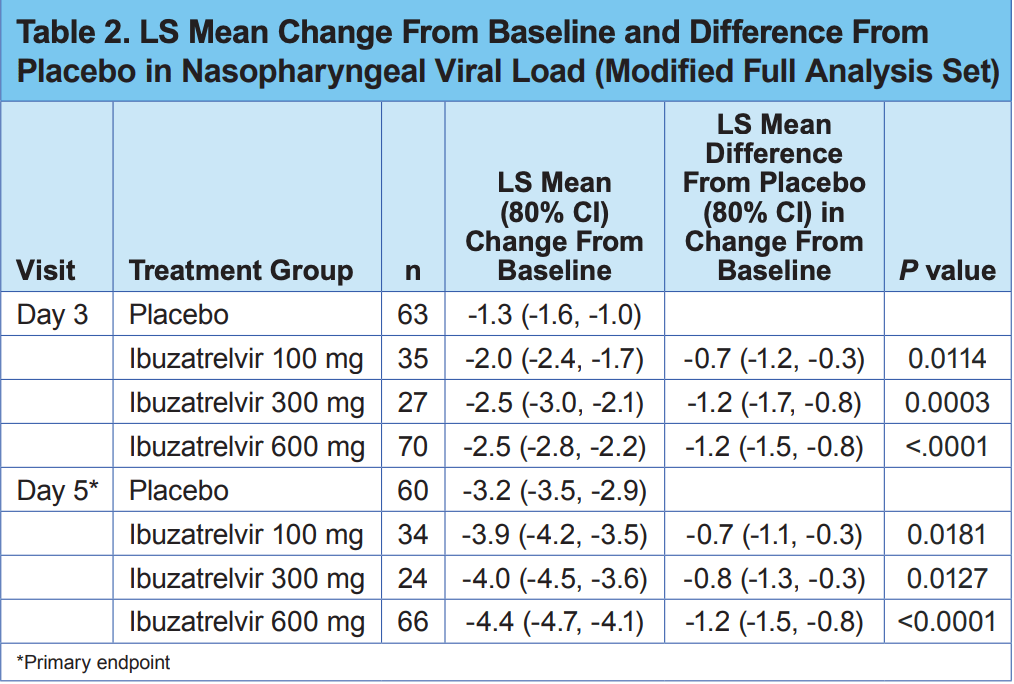
RCT 240 outpatients showing significant viral load reduction with ibuzatrelvir (an oral SARS-CoV-2 Mpro inhibitor) compared to placebo. The study enrolled non-hospitalized adults aged 18-65 with symptomatic COVID-19 (≤5 days) and positive rapid antigen test, excluding those with obesity, smoking, chronic conditions, or immunocompromised status. Patients were randomized 1:1:2:2 to receive 100mg, 300mg, or 600mg ibuzatrelvir or placebo twice daily for 5 days. There were dose-dependent decreases in viral load at days 3 and 5 compared to placebo. The trial has limited generalizability due to strict exclusion criteria that eliminated many high-risk patients who would typically be candidates for antiviral therapy.
Standard of Care (SOC) for COVID-19 in the study country,
the USA, is very poor with very low average efficacy for approved treatments1.
Only expensive, high-profit treatments were approved for early treatment. Low-cost treatments were excluded, reducing the probability of early treatment due to access and cost barriers, and eliminating complementary and synergistic benefits seen with many low-cost treatments.
|
viral load, 27.3% lower, relative load 0.73, p < 0.001, treatment 66, control 60, 600mg, day 5.
|
|
viral load, 20.0% lower, relative load 0.80, p = 0.01, treatment 24, control 60, 300mg, day 5.
|
|
viral load, 17.9% lower, relative load 0.82, p = 0.02, treatment 34, control 60, 100mg, day 5.
|
|
viral load, 48.0% lower, relative load 0.52, p < 0.001, treatment 70, control 63, 600mg, day 3.
|
|
viral load, 48.0% lower, relative load 0.52, p < 0.001, treatment 27, control 63, 300mg, day 3.
|
|
viral load, 35.0% lower, relative load 0.65, p = 0.01, treatment 35, control 63, 100mg, day 3.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Mortezavi et al., 2 Nov 2024, Double Blind Randomized Controlled Trial, placebo-controlled, USA, peer-reviewed, 13 authors, study period 24 May, 2023 - 8 September, 2023, trial NCT05799495 (history).
Contact: mahta.mortezavi@pfizer.com, niki.alami@pfizer.com.
Virologic Response and Safety of Ibuzatrelvir, a Novel SARS-cov-2 Antiviral, in Adults With COVID-19
doi:10.1093/cid/ciae5291
Background: Despite effective vaccines and treatments for COVID-19, clinical burden persists. An unmet need exists for additional effective agents with safety profiles allowing use across a broad population. Ibuzatrelvir is an orally bioavailable SARS-CoV-2 M pro inhibitor that has demonstrated in vitro antiviral activity and low potential for safety concerns, including drug-drug interactions. Methods: This phase 2b, double-blind, randomized clinical trial enrolled US adults aged 18-<65 years with symptomatic COVID-19 and no risk factors for severe disease. Participants were randomized 1:1:2:2 to receive 100, 300, or 600 mg ibuzatrelvir or placebo orally twice daily for 5 days. Nasopharyngeal specimens were collected on Days 1 (baseline), 3, 5, 10, 14, and 21; adverse
Conflict of Interest Disclosures: All authors are employees of Pfizer and may hold stock or stock options.
References
Aggarwal, Molina, Beaty, Real-world use of nirmatrelvir-ritonavir in outpatients with COVID-19 during the era of omicron variants including BA.4 and BA.5 in Colorado, USA: a retrospective cohort study, Lancet Infect Dis, doi:10.1016/S1473-3099(23)00011-7
Akinosoglou, Rigopoulos, Schinas, Remdesivir use in the real-world setting: an overview of available evidence, Viruses, doi:10.3390/v15051167
Allerton, Arcari, Aschenbrenner, A Second-Generation Oral SARS-CoV-2 Main Protease Inhibitor Clinical Candidate for the Treatment of COVID-19, J Med Chem, doi:10.1021/acs.jmedchem.3c02469
Berg, Zhen, Lucic, Development of the RealTime SARS-CoV-2 quantitative Laboratory Developed Test and correlation with viral culture as a measure of infectivity, J Clin Virol, doi:10.1016/j.jcv.2021.104945
Borroto-Esoda, Wilfret, Tong, Plummer, Kearney et al., SARS-CoV-2 viral dynamics in a placebo-controlled phase 2 study of patients infected with the SARS-CoV-2 Omicron variant and treated with pomotrelvir, Microbiol Spectr, doi:10.1128/spectrum.02980-23
Boyapati, Wipperman, Ehmann, Baseline severe acute respiratory syndrome viral load is associated with coronavirus disease 2019 severity and clinical outcomes: post hoc analyses of a phase 2/3 trial, J Infect Dis, doi:10.1093/infdis/jiab445
Brnic, Buric, Marcec, Likic, The relationship between vaccine acceptance and COVID-19 mortality in Europe: A Cross-Country analysis of public opinion and Epidemiological data, Vaccine X, doi:10.1016/j.jvacx.2023.100391
Cao, Wang, Lu, Oral simnotrelvir for adult patients with mild-to-moderate Covid-19, N Engl J Med, doi:10.1056/NEJMoa2301425
Gerhart, Draica, Benigno, Real-world evidence of the top 100 prescribed drugs in the USA and their potential for drug interactions with nirmatrelvir; ritonavir, AAPS J, doi:10.1208/s12248-023-00832-3
Hammond, Leister-Tebbe, Gardner, Oral nirmatrelvir for high-risk, nonhospitalized adults with COVID-19, N Engl J Med, doi:10.1056/NEJMoa2118542
Hanrath, Payne, Duncan, Prior SARS-CoV-2 infection is associated with protection against symptomatic reinfection, J Infect, doi:10.1016/j.jinf.2020.12.023
Harrington, Cong, Troy, Evaluation of SARS-CoV-2 RNA rebound after nirmatrelvir/ritonavir treatment in randomized, double-blind, placebo-controlled trials -United States and International Sites, 2021-2022, MMWR Morb Mortal Wkly Rep, doi:10.15585/mmwr.mm7251a2
Jain, Serisier, Lorgelly, The real-world impact of vaccination on COVID-19 cases during Europe's Fourth Wave, Int J Public Health, doi:10.3389/ijph.2022.1604793
Jones, Manrique, Stone, Estimates of SARS-CoV-2 seroprevalence and incidence of primary SARS-CoV-2 infections among blood donors, by COVID-19 vaccination status -United States, April 2021-September 2022, MMWR Morb Mortal Wkly Rep, doi:10.15585/mmwr.mm7222a3
Li, Hilgenfeld, Whitley, Clercq, Therapeutic strategies for COVID-19: progress and lessons learned, Nat Rev Drug Discov, doi:10.1038/s41573-023-00672-y
Magleby, Westblade, Trzebucki, Impact of severe acute respiratory syndrome coronavirus 2 viral load on risk of intubation and mortality among hospitalized patients with coronavirus disease 2019, Clin Infect Dis, doi:10.1093/cid/ciaa851
Monach, Anand, Fillmore, La, Branch-Elliman, Underuse of antiviral drugs to prevent progression to severe COVID-19 -Veterans Health Administration, March-September 2022, MMWR Morb Mortal Wkly Rep, doi:10.15585/mmwr.mm7303a2
Mukae, Yotsuyanagi, Ohmagari, Efficacy and safety of ensitrelvir in patients with mild-to-moderate coronavirus disease 2019: the phase 2b part of a randomized, placebo-controlled, phase 2/3 study, Clin Infect Dis, doi:10.1093/cid/ciac933
Sahasrabudhe, Mortezavi, Toussi, The SARS-CoV-2 Mpro Inhibitor Ibuzatrelvir is a Substrate but not an Inducer nor Inhibitor of CYP3A, American College of Clinical Pharmacology
Satapathy, Kumar, Mehta, Global spread of COVID-19's JN.1 variant: implications and public health responses, New Microbes New Infect, doi:10.1016/j.nmni.2024.101225
Singh, Toussi, Hackman, Innovative randomized phase I study and dosing regimen selection to accelerate and inform pivotal COVID-19 trial of nirmatrelvir, Clin Pharmacol Ther, doi:10.1002/cpt.2603
Smith, Lambrou, Patel, SARS-CoV-2 rebound with and without use of COVID-19 oral antivirals, MMWR Morb Mortal Wkly Rep, doi:10.15585/mmwr.mm7251a1
Standing, Buggiotti, Guerra-Assuncao, Randomized controlled trial of molnupiravir SARS-CoV-2 viral and antibody response in at-risk adult outpatients, Nat Commun, doi:10.1038/s41467-024-45641-0
Tuttle, Discovery of PF-07817883: A next generation oral protease inhibitor for the treatment of COVID-19, American Chemical Society Fall
Wagenhauser, Knies, Hofmann, Virus variant-specific clinical performance of SARS coronavirus two rapid antigen tests in point-of-care use, from November 2020 to January 2022, Clin Microbiol Infect, doi:10.1016/j.cmi.2022.08.006
Wang, Zhang, Du, Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial, Lancet, doi:10.1016/S0140-6736(20)31022-9
Westblade, Brar, Pinheiro, SARS-CoV-2 viral load predicts mortality in patients with and without cancer who are hospitalized with COVID-19, Cancer Cell, doi:10.1016/j.ccell.2020.09.007
Yamana, Galanti, Pei, The impact of COVID-19 vaccination in the US: averted burden of SARS-COV-2-related cases, hospitalizations and deaths, PLoS One, doi:10.1371/journal.pone.0275699
DOI record:
{
"DOI": "10.1093/cid/ciae529",
"ISSN": [
"1058-4838",
"1537-6591"
],
"URL": "http://dx.doi.org/10.1093/cid/ciae529",
"abstract": "<jats:title>Abstract</jats:title>\n <jats:sec>\n <jats:title>Background</jats:title>\n <jats:p>Despite effective vaccines and treatments for COVID-19, clinical burden persists. An unmet need exists for additional effective agents with safety profiles allowing use across a broad population. Ibuzatrelvir is an orally bioavailable SARS-CoV-2 Mpro inhibitor that has demonstrated in vitro antiviral activity and low potential for safety concerns, including drug-drug interactions.</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>Methods</jats:title>\n <jats:p>This phase 2b, double-blind, randomized clinical trial enrolled US adults aged 18‒&lt;65 years with symptomatic COVID-19 and no risk factors for severe disease. Participants were randomized 1:1:2:2 to receive 100, 300, or 600 mg ibuzatrelvir or placebo orally twice daily for 5 days. Nasopharyngeal specimens were collected on Days 1 (baseline), 3, 5, 10, 14, and 21; adverse events (AEs) were recorded through Day 33. The primary endpoint was change in SARS-CoV-2 RNA level (viral load [VL]) from baseline to Day 5 among participants with baseline VL ≥4 log10 copies/mL.</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>Results</jats:title>\n <jats:p>Of 240 enrollees, 237 received ≥1 dose and 199 were included in the primary analysis. Placebo-adjusted least squares mean (80% CI) change from baseline in VL at Day 5 was significant across all doses: 100 mg, ‒0.7 (‒1.1, ‒0.3) log10 copies/mL, P=0.02; 300 mg, ‒0.8 (‒1.3, ‒0.3) log10 copies/mL, P=0.01; and 600 mg, ‒1.2 (‒1.5, ‒0.8) log10 copies/mL, P&lt;0.0001. AEs occurred in similar percentages of participants across groups. No deaths from any cause or treatment-related serious AEs occurred through Day 33, and no participants reported dysgeusia.</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>Conclusions</jats:title>\n <jats:p>All 3 ibuzatrelvir doses were associated with robust antiviral activity and an acceptable safety profile, supporting continued clinical development.</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>Trial Registration</jats:title>\n <jats:p>Clinicaltrials.gov identifier: NCT05799495</jats:p>\n </jats:sec>",
"author": [
{
"affiliation": [
{
"name": "Pfizer Inc , New York, NY ,",
"place": [
"USA"
]
}
],
"family": "Mortezavi",
"given": "Mahta",
"sequence": "first"
},
{
"affiliation": [
{
"name": "Pfizer Inc , Cambridge, MA ,",
"place": [
"USA"
]
}
],
"family": "Sloan",
"given": "Abigail",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Pfizer Inc , Cambridge, MA ,",
"place": [
"USA"
]
}
],
"family": "Singh",
"given": "Ravi Shankar P",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Pfizer Inc , Cambridge, MA ,",
"place": [
"USA"
]
}
],
"family": "Chen",
"given": "Luke F",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Pfizer Inc , Collegeville, PA ,",
"place": [
"USA"
]
}
],
"family": "Kim",
"given": "Jin Hyang",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Pfizer Inc , Cambridge, MA ,",
"place": [
"USA"
]
}
],
"family": "Shojaee",
"given": "Negin",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Pfizer Inc , Pearl River, NY ,",
"place": [
"USA"
]
}
],
"family": "Toussi",
"given": "Sima S",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Pfizer Inc , Groton, CT ,",
"place": [
"USA"
]
}
],
"family": "Prybylski",
"given": "John",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Pfizer Inc , Cambridge, MA ,",
"place": [
"USA"
]
}
],
"family": "Baniecki",
"given": "Mary Lynn",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Pfizer Inc , Groton, CT ,",
"place": [
"USA"
]
}
],
"family": "Bergman",
"given": "Arthur",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Pfizer Inc , Cambridge, MA ,",
"place": [
"USA"
]
}
],
"family": "Banerjee",
"given": "Anindita",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Pfizer Inc , Cambridge, MA ,",
"place": [
"USA"
]
}
],
"family": "Allerton",
"given": "Charlotte",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Pfizer Inc , New York, NY ,",
"place": [
"USA"
]
}
],
"family": "Alami",
"given": "Negar Niki",
"sequence": "additional"
}
],
"container-title": "Clinical Infectious Diseases",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2024,
11,
1
]
],
"date-time": "2024-11-01T22:22:57Z",
"timestamp": 1730499777000
},
"deposited": {
"date-parts": [
[
2024,
11,
1
]
],
"date-time": "2024-11-01T22:22:57Z",
"timestamp": 1730499777000
},
"indexed": {
"date-parts": [
[
2024,
11,
2
]
],
"date-time": "2024-11-02T04:14:23Z",
"timestamp": 1730520863973,
"version": "3.28.0"
},
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2024,
11,
2
]
]
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by-nc-nd/4.0/",
"content-version": "am",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2024,
11,
1
]
],
"date-time": "2024-11-01T00:00:00Z",
"timestamp": 1730419200000
}
}
],
"link": [
{
"URL": "https://academic.oup.com/cid/advance-article-pdf/doi/10.1093/cid/ciae529/60298252/ciae529.pdf",
"content-type": "application/pdf",
"content-version": "am",
"intended-application": "syndication"
},
{
"URL": "https://academic.oup.com/cid/advance-article-pdf/doi/10.1093/cid/ciae529/60298252/ciae529.pdf",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "286",
"original-title": [],
"prefix": "10.1093",
"published": {
"date-parts": [
[
2024,
11,
2
]
]
},
"published-online": {
"date-parts": [
[
2024,
11,
2
]
]
},
"publisher": "Oxford University Press (OUP)",
"reference-count": 0,
"references-count": 0,
"relation": {},
"resource": {
"primary": {
"URL": "https://academic.oup.com/cid/advance-article/doi/10.1093/cid/ciae529/7863440"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Virologic Response and Safety of Ibuzatrelvir, a Novel SARS-CoV-2 Antiviral, in Adults With COVID-19",
"type": "journal-article"
}