Real-world use of nirmatrelvir–ritonavir in outpatients with COVID-19 during the era of omicron variants including BA.4 and BA.5 in Colorado, USA: a retrospective cohort study
et al., The Lancet Infectious Diseases, doi:10.1016/S1473-3099(23)00011-7, Feb 2023
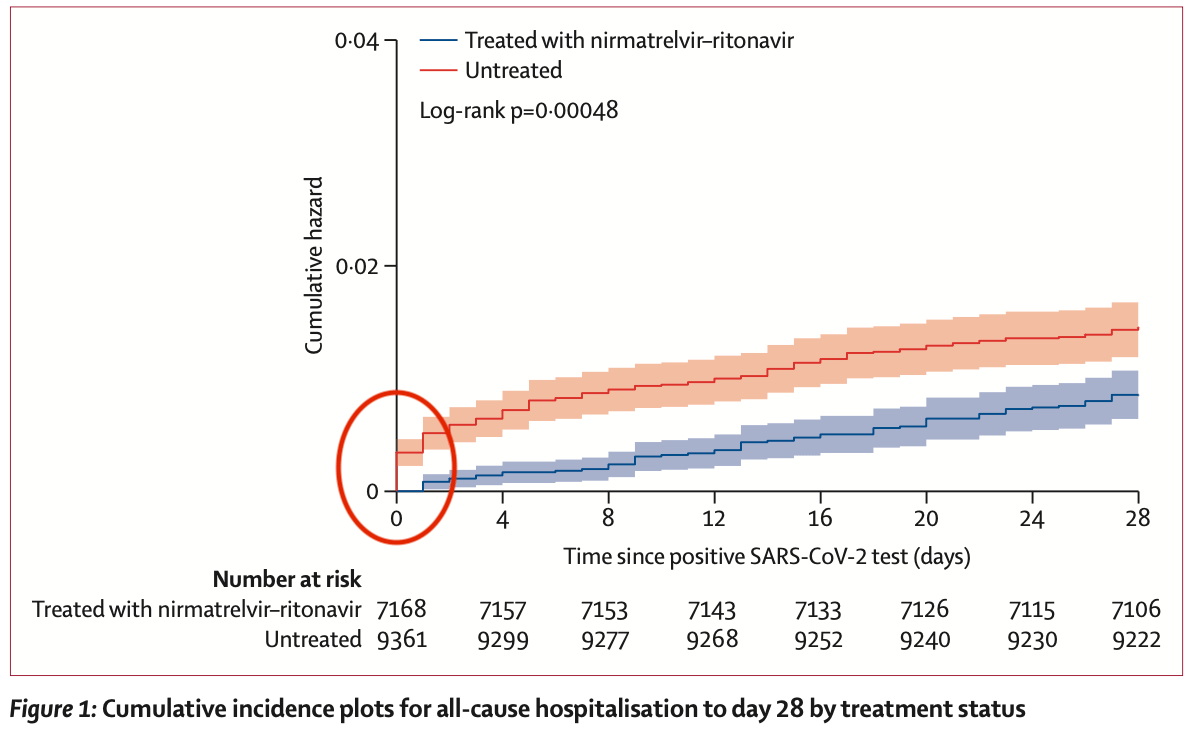
Retrospective 28,167 patients in the USA demonstrating confounding. The large difference shown in Figure 1 at day 0 indicates that the groups are not comparable (32 control hospitalizations versus 0 for paxlovid at day 0) and suggests immortal time bias / confounding by indication. For example, patients in more serious condition may be sent for admission rather than to the pharmacy for paxlovid. ~60% of the difference between the groups at day 28 exists at day 0, and close to 100% by day 4. Confounding may also arise due to inclusion of contraindicated patients in the control group, only partially investigated in sensitivity analysis. Patients that seek out paxlovid may also differ in risk to those that do not.
Authors also note that "The post-hoc sensitivity analysis derived from a cohort of only patients with an observed SARS-CoV-2 postitive [sic] test date was 70% smaller than the primary cohort, and the point estimate for a nirmatrelvir-ritonavir association with reduced 28-day hospitalisation did not reach statistical significance."
Resistance. Variants may be resistant to paxlovid1-8. Use may promote the emergence of variants that weaken host immunity and potentially contribute to long COVID9. Confounding by contraindication. Hoertel et al. find that over 50% of patients that died had a contraindication for the use of Paxlovid10. Retrospective studies that do not exclude contraindicated patients may significantly overestimate efficacy. Black box warning. The FDA notes that severe, life-threatening, and/or fatal adverse reactions due to drug interactions have been reported in patients treated with paxlovid11. Kidney and liver injury. Studies show significantly increased risk of acute kidney injury12 and liver injury13,14. Viral rebound. Studies show significantly increased risk of replication-competent viral rebound15-17.
1.
Zhou et al., Nirmatrelvir-resistant SARS-CoV-2 variants with high fitness in an infectious cell culture system, Science Advances, doi:10.1126/sciadv.add7197.
2.
Moghadasi et al., Rapid resistance profiling of SARS-CoV-2 protease inhibitors, npj Antimicrobials and Resistance, doi:10.1038/s44259-023-00009-0.
3.
Jochmans et al., The Substitutions L50F, E166A, and L167F in SARS-CoV-2 3CLpro Are Selected by a Protease Inhibitor In Vitro and Confer Resistance To Nirmatrelvir, mBio, doi:10.1128/mbio.02815-22.
4.
Lopez et al., SARS-CoV-2 Resistance to Small Molecule Inhibitors, Current Clinical Microbiology Reports, doi:10.1007/s40588-024-00229-6.
5.
Zvornicanin et al., Molecular Mechanisms of Drug Resistance and Compensation in SARS-CoV-2 Main Protease: The Interplay Between E166 and L50, bioRxiv, doi:10.1101/2025.01.24.634813.
6.
Vukovikj et al., Impact of SARS-CoV-2 variant mutations on susceptibility to monoclonal antibodies and antiviral drugs: a non-systematic review, April 2022 to October 2024, Eurosurveillance, doi:10.2807/1560-7917.ES.2025.30.10.2400252.
7.
Deschenes et al., Functional and structural characterization of treatment-emergent nirmatrelvir resistance mutations at low frequencies in the main protease (Mpro) reveals a unique evolutionary route for SARS-CoV-2 to gain resistance, The Journal of Infectious Diseases, doi:10.1093/infdis/jiaf294.
8.
Zhou (B) et al., SARS-CoV-2 Mpro inhibitor ensitrelvir: asymmetrical cross-resistance with nirmatrelvir and emerging resistance hotspots, Emerging Microbes & Infections, doi:10.1080/22221751.2025.2552716.
9.
Thomas et al., Nirmatrelvir-Resistant Mutations in SARS-CoV-2 Mpro Enhance Host Immune Evasion via Cleavage of NF-κB Essential Modulator, bioRxiv, doi:10.1101/2024.10.18.619137.
10.
Hoertel et al., Prevalence of Contraindications to Nirmatrelvir-Ritonavir Among Hospitalized Patients With COVID-19 at Risk for Progression to Severe Disease, JAMA Network Open, doi:10.1001/jamanetworkopen.2022.42140.
11.
FDA, Fact sheet for healthcare providers: emergency use authorization for paxlovid, www.fda.gov/media/155050/download.
12.
Kamo et al., Association of Antiviral Drugs for the Treatment of COVID-19 With Acute Renal Failure, In Vivo, doi:10.21873/invivo.13637.
13.
Wang et al., Development and validation of a nomogram to assess the occurrence of liver dysfunction in patients with COVID-19 pneumonia in the ICU, BMC Infectious Diseases, doi:10.1186/s12879-025-10684-1.
14.
Siby et al., Temporal Trends in Serious Adverse Events Associated with Oral Antivirals During the COVID-19 Pandemic: Insights from the FAERS Database (2020–2023), Open Forum Infectious Diseases, doi:10.1093/ofid/ofaf695.1825.
15.
Edelstein et al., SARS-CoV-2 virologic rebound with nirmatrelvir-ritonavir therapy, medRxiv, doi:10.1101/2023.06.23.23288598.
Aggarwal et al., 9 Feb 2023, retrospective, USA, peer-reviewed, 10 authors.
Real-world use of nirmatrelvir–ritonavir in outpatients with COVID-19 during the era of omicron variants including BA.4 and BA.5 in Colorado, USA: a retrospective cohort study
The Lancet Infectious Diseases, doi:10.1016/s1473-3099(23)00011-7
Background Nirmatrelvir is a protease inhibitor with in-vitro activity against SARS-CoV-2, and ritonavir-boosted nirmatrelvir can reduce the risk of progression to severe COVID-19 among individuals at high risk infected with delta and early omicron variants. However, less is known about the effectiveness of nirmatrelvir-ritonavir during more recent BA.2, BA2.12.1, BA.4, and BA.5 omicron variant surges. We used our real-world data platform to evaluate the effect of nirmatrelvir-ritonavir treatment on 28-day hospitalisation, mortality, and emergency department visits among outpatients with early symptomatic COVID-19 during a SARS-CoV-2 omicron (BA.2, BA2.12.1, BA.4, and BA.5) predominant period in Colorado, USA.
Methods We did a propensity-matched, retrospective, observational cohort study of non-hospitalised adult patients infected with SARS-CoV-2 between March 26 and Aug 25, 2022, using records from a statewide health system in Colorado. We obtained data from the electronic health records of University of Colorado Health, the largest health system in Colorado, with 13 hospitals and 141 000 annual hospital admissions, and with numerous ambulatory sites and affiliated pharmacies around the state. Included patients had a positive SARS-CoV-2 test or nirmatrelvir-ritonavir medication order. Exclusion criteria were an order for or administration of other SARS-CoV-2 treatments within 10 days of a positive SARS-CoV-2 test, hospitalisation at the time of positive SARS-CoV-2 test, and positive SARS-CoV-2 test more than 10 days before a nirmatrelvir-ritonavir order. We propensity score matched patients treated with nirmatrelvir-ritonavir with untreated patients. The primary outcome was 28-day all-cause hospitalisation. Findings Among 28 167 patients infected with SARS-CoV-2 between March 26 and Aug 25, 2022, 21 493 met the study inclusion criteria. 9881 patients received treatment with nirmatrelvir-ritonavir and 11 612 were untreated. Nirmatrelvir-ritonavir treatment was associated with reduced 28-day all-cause hospitalisation compared with no antiviral treatment (61 [0•9%] of 7168 patients vs 135 [1•4%] of 9361 patients, adjusted odds ratio (OR) 0•45 [95% CI 0•33-0•62]; p<0•0001). Nirmatrelvir-ritonavir treatment was also associated with reduced 28-day all-cause mortality (two [<0•1%] of 7168 patients vs 15 [0•2%] of 9361 patients; adjusted OR 0•15 [95% CI 0•03-0•50]; p=0•0010). Using subsequent emergency department visits as a surrogate for clinically significant relapse, we observed a decrease after nirmatrelvir-ritonavir treatment (283 [3•9%] of 7168 patients vs 437 [4•7%] of 9361 patients; adjusted OR 0•74 [95% CI 0•63-0•87]; p=0•0002). Interpretation Real-world evidence reported during a BA.2, BA2.12.1, BA.4, and BA.5 omicron surge showed an association between nirmatrelvir-ritonavir treatment and reduced 28-day all-cause hospitalisation, all-cause mortality, and visits to the emergency department. With results that are among the first to..
References
Aggarwal, Beaty, Bennett, Change in effectiveness of sotrovimab for preventing hospitalization and mortality for at-risk COVID-19 outpatients during an omicron BA.1 and BA.1.1-predominant phase, Int J Infect Dis, doi:10.1016/j.ijid.2022.10.002
Aggarwal, Beaty, Bennett, Real-world evidence of the neutralizing monoclonal antibody sotrovimab for preventing hospitalization and mortality in COVID-19 outpatients, J Infect Dis
Allerton, Anderson, All sensitivity analyses were fit using Firth's bias-reducing logistic regression, with 28-day all-cause hospitalisation as the outcome, and were adjusted for all covariates in the primary analysis. Table 3: Primary and sensitivity analyses for all-cause hospitalisation at 28 days References 1 Owen DR, Science
Arbel, Sagy, Hoshen, Nirmatrelvir use and severe COVID-19 outcomes during the omicron surge, N Engl J Med
Austin, An introduction to propensity score methods for reducing the effects of confounding in observational studies, Multivariate Behav Res
Austin, Optimal caliper widths for propensity-score matching when estimating differences in means and differences in proportions in observational studies, Pharm Stat
Austin, Statistical criteria for selecting the optimal number of untreated subjects matched to each treated subject when using many-to-one matching on the propensity score, Am J Epidemiol
Coulson, Adams, Gray, Evans, COVID-19 "rebound" associated with nirmatrelvir/ritonavir pre-hospital therapy, J Infect
Dryden-Peterson, Kim, Kim, Nirmatrelvir plus ritonavir for early COVID-19 and hospitalization in a large US health system, medRxiv, doi:10.1101/2022.06.14.22276393/
Ganatra, Dani, Ahmad, Oral nirmatrelvir and ritonavir in non-hospitalized vaccinated patients with COVID-19, Clin Infect Dis, doi:10.1093/cid/ciac673
Hammond, Leister-Tebbe, Gardner, Oral nirmatrelvir for high-risk, nonhospitalized adults with COVID-19, N Engl J Med
Heinze, Ploner, Dunkler, Southworth, Jiricka, logistf: Firth's bias-reduced logistic regression
Heinze, Schemper, A solution to the problem of separation in logistic regression, Stat Med
Ho, Imai, King, Stuart, Matching as nonparametric preprocessing for reducing model dependence in parametric causal inference, Polit Anal
Lüdecke, Makowski, Ben-Shachar, performance: an R package for assessment, comparison and testing of statistical models, J Open Source Softw
Malden, Hong, Lewin, Hospitalization and emergency department encounters for COVID-19 after paxlovid treatment-California, December 2021-May 2022, MMWR Morb Mortal Wkly Rep
Najjar-Debbiny, Gronich, Weber, Effectiveness of paxlovid in reducing severe COVID-19 and mortality in high risk patients, Clin Infect Dis, doi:10.1093/cid/ciac443
Nguyen, Collins, Spence, Double-adjustment in propensity score matching analysis: choosing a threshold for considering residual imbalance, BMC Med Res Methodol
Nyberg, Ferguson, Nash, Comparative analysis of the risks of hospitalisation and death associated with SARS-CoV-2 omicron (B.1.1.529) and delta (B.1.617.2) variants in England: a cohort study, Lancet
Puhr, Heinze, Nold, Lusa, Geroldinger, Firth's logistic regression with rare events: accurate effect estimates and predictions?, Stat Med
Razonable, Horo, Hanson, Comparable outcomes for bebtelovimab and ritonavir-boosted nirmatrelvir treatment in high-risk patients with coronavirus disease-2019 during severe acute respiratory syndrome coronavirus 2 BA.2 omicron epoch, J Infect Dis
Shah, Joyce, Plumb, Paxlovid associated with decreased hospitalization rate among adults with COVID-19-United States, April-September 2022, MMWR Morb Mortal Wkly Rep
Takashita, Yamayoshi, Simon, Efficacy of antibodies and antiviral drugs against omicron BA.2.12.1, BA.4, and BA.5 subvariants, N Engl J Med
Wong, Au, Lau, Lau, Cowling et al., Real-world effectiveness of early molnupiravir or nirmatrelvirritonavir in hospitalised patients with COVID-19 without supplemental oxygen requirement on admission during Hong Kong's omicron BA.2 wave: a retrospective cohort study, Lancet Infect Dis
Wynia, Beaty, Bennett, Real-world evidence of neutralizing monoclonal antibodies for preventing hospitalization and mortality in COVID-19 outpatients, Chest, doi:10.1016/j.chest.2022.10.020
Yip, Lui, Lai, Impact of the use of oral antiviral agents on the risk of hospitalization in community COVID-19 patients, Clin Infect Dis, doi:10.1093/cid/ciac687
Zhou, Kelly, Liang, Real-world effectiveness of nirmatrelvir/ritonavir in preventing hospitalization among patients with COVID-19 at high risk for severe disease in the United States: a nationwide population-based cohort study, medRxiv, doi:10.1101/2022.09.13.22279908