Real-World Effectiveness of Nirmatrelvir/Ritonavir in Preventing Hospitalization Among Patients With COVID-19 at High Risk for Severe Disease in the United States: A Nationwide Population-Based Cohort Study
et al., medRxiv, doi:10.1101/2022.09.13.22279908, Sep 2022
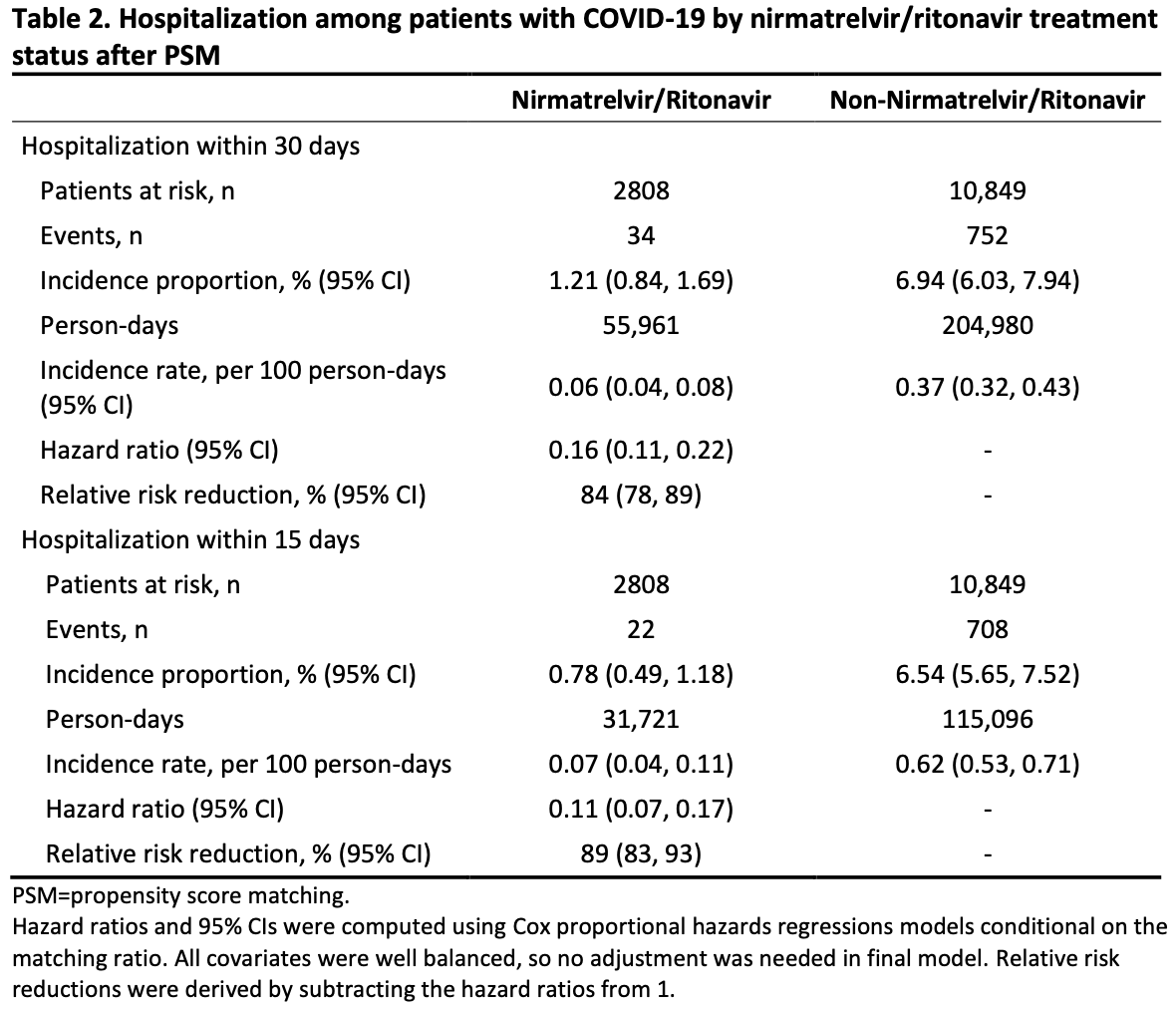
Pfizer retrospective 2,811 high risk COVID-19 patients treated with paxlovid in the US, and 10,849 matched controls, showing lower risk of mortality and hospitalization with treatment.
Resistance. Variants may be resistant to paxlovid1-8. Use may promote the emergence of variants that weaken host immunity and potentially contribute to long COVID9. Confounding by contraindication. Hoertel et al. find that over 50% of patients that died had a contraindication for the use of Paxlovid10. Retrospective studies that do not exclude contraindicated patients may significantly overestimate efficacy. Black box warning. The FDA notes that severe, life-threatening, and/or fatal adverse reactions due to drug interactions have been reported in patients treated with paxlovid11. Kidney and liver injury. Studies show significantly increased risk of acute kidney injury12 and liver injury13,14. Viral rebound. Studies show significantly increased risk of replication-competent viral rebound15-17.
Standard of Care (SOC) for COVID-19 in the study country,
the USA, is very poor with very low average efficacy for approved treatments18.
Only expensive, high-profit treatments were approved for early treatment. Low-cost treatments were excluded, reducing the probability of early treatment due to access and cost barriers, and eliminating complementary and synergistic benefits seen with many low-cost treatments.
|
risk of death, 73.0% lower, RR 0.27, p < 0.001, treatment 7 of 2,808 (0.2%), control 100 of 10,849 (0.9%), NNT 149, propensity score matching, day 30.
|
|
risk of death, 75.2% lower, RR 0.25, p < 0.001, treatment 5 of 2,808 (0.2%), control 78 of 10,849 (0.7%), NNT 185, propensity score matching, day 15.
|
|
risk of hospitalization, 84.0% lower, HR 0.16, p < 0.001, treatment 34 of 2,808 (1.2%), control 752 of 10,849 (6.9%), NNT 17, propensity score matching, Cox proportional hazards, day 30.
|
|
risk of hospitalization, 89.0% lower, HR 0.11, p < 0.001, treatment 22 of 2,808 (0.8%), control 708 of 10,849 (6.5%), NNT 17, propensity score matching, Cox proportional hazards, day 15.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
1.
Zhou et al., Nirmatrelvir-resistant SARS-CoV-2 variants with high fitness in an infectious cell culture system, Science Advances, doi:10.1126/sciadv.add7197.
2.
Moghadasi et al., Rapid resistance profiling of SARS-CoV-2 protease inhibitors, npj Antimicrobials and Resistance, doi:10.1038/s44259-023-00009-0.
3.
Jochmans et al., The Substitutions L50F, E166A, and L167F in SARS-CoV-2 3CLpro Are Selected by a Protease Inhibitor In Vitro and Confer Resistance To Nirmatrelvir, mBio, doi:10.1128/mbio.02815-22.
4.
Lopez et al., SARS-CoV-2 Resistance to Small Molecule Inhibitors, Current Clinical Microbiology Reports, doi:10.1007/s40588-024-00229-6.
5.
Zvornicanin et al., Molecular Mechanisms of Drug Resistance and Compensation in SARS-CoV-2 Main Protease: The Interplay Between E166 and L50, bioRxiv, doi:10.1101/2025.01.24.634813.
6.
Vukovikj et al., Impact of SARS-CoV-2 variant mutations on susceptibility to monoclonal antibodies and antiviral drugs: a non-systematic review, April 2022 to October 2024, Eurosurveillance, doi:10.2807/1560-7917.ES.2025.30.10.2400252.
7.
Deschenes et al., Functional and structural characterization of treatment-emergent nirmatrelvir resistance mutations at low frequencies in the main protease (Mpro) reveals a unique evolutionary route for SARS-CoV-2 to gain resistance, The Journal of Infectious Diseases, doi:10.1093/infdis/jiaf294.
8.
Zhou (B) et al., SARS-CoV-2 Mpro inhibitor ensitrelvir: asymmetrical cross-resistance with nirmatrelvir and emerging resistance hotspots, Emerging Microbes & Infections, doi:10.1080/22221751.2025.2552716.
9.
Thomas et al., Nirmatrelvir-Resistant Mutations in SARS-CoV-2 Mpro Enhance Host Immune Evasion via Cleavage of NF-κB Essential Modulator, bioRxiv, doi:10.1101/2024.10.18.619137.
10.
Hoertel et al., Prevalence of Contraindications to Nirmatrelvir-Ritonavir Among Hospitalized Patients With COVID-19 at Risk for Progression to Severe Disease, JAMA Network Open, doi:10.1001/jamanetworkopen.2022.42140.
11.
FDA, Fact sheet for healthcare providers: emergency use authorization for paxlovid, www.fda.gov/media/155050/download.
12.
Kamo et al., Association of Antiviral Drugs for the Treatment of COVID-19 With Acute Renal Failure, In Vivo, doi:10.21873/invivo.13637.
13.
Wang et al., Development and validation of a nomogram to assess the occurrence of liver dysfunction in patients with COVID-19 pneumonia in the ICU, BMC Infectious Diseases, doi:10.1186/s12879-025-10684-1.
14.
Siby et al., Temporal Trends in Serious Adverse Events Associated with Oral Antivirals During the COVID-19 Pandemic: Insights from the FAERS Database (2020–2023), Open Forum Infectious Diseases, doi:10.1093/ofid/ofaf695.1825.
15.
Edelstein et al., SARS-CoV-2 virologic rebound with nirmatrelvir-ritonavir therapy, medRxiv, doi:10.1101/2023.06.23.23288598.
16.
Shah et al., SARS-CoV-2 infectious shedding and rebound among adults with and without oral antiviral use: two case-ascertained prospective household studies, The Lancet Microbe, doi:10.1016/j.lanmic.2025.101227.
Zhou et al., 14 Sep 2022, retrospective, USA, preprint, median age 62.0, 9 authors, study period 22 December, 2021 - 8 June, 2022.
Real-World Effectiveness of Nirmatrelvir/Ritonavir in Preventing Hospitalization Among Patients With COVID-19 at High Risk for Severe Disease in the United States: A Nationwide Population-Based Cohort Study
doi:10.1101/2022.09.13.22279908
Objectives: The aim of this analysis was to describe nirmatrelvir/ritonavir real-world effectiveness in preventing hospitalization among high-risk US COVID-19 patients during SARS-CoV-2 Omicron predominance. Design: An ongoing population-based cohort study with retrospective and prospective collection of electronic healthcare data in the United States. Methods: Data for this analysis were collected from the US Optum® de-identified COVID-19 Electronic Health Record (EHR) dataset during December 22, 2021−June 8, 2022. Key eligibility criteria for inclusion in the database analysis were ≥12-years-old; positive SARS-CoV-2 test, COVID-19 diagnosis, or nirmatrelvir/ritonavir prescription; and high risk of severe COVID-19 based on demographic/clinical characteristics. Potential confounders between groups were balanced using propensity score matching (PSM). Immortal time bias was addressed. Outcome measures: Hospitalization rates within 30 (primary analysis) or 15 (sensitivity analysis) days from COVID-19 diagnosis overall and within subgroups were evaluated. Results: Before PSM, the nirmatrelvir/ritonavir group (n=2811) was less racially diverse, older, and had higher COVID-19 vaccination rates and a greater number of comorbidities than the nonnirmatrelvir/ritonavir group (n=194,542). Baseline characteristics were well balanced across groups (n=2808 and n=10,849, respectively) after PSM. Incidence of hospitalization (95% CI) within 30 days was 1.21% (0.84%−1.69%) for the nirmatrelvir/ritonavir group and 6.94% (6.03%−7.94%) for the nonnirmatrelvir/ritonavir group, with a hazard ratio (95% CI) of 0.16 (0.11−0.22; 84% relative risk reduction). Incidence within 15 days was 0.78% (0.49%−1.18%) for the nirmatrelvir/ritonavir group and 6.54% (5.65%−7.52%) for the non-nirmatrelvir/ritonavir group; hazard ratio 0.11 (0.07−0.17; 89% relative risk reduction). Nirmatrelvir/ritonavir was effective in African American patients (hazard ratio, 0.35 [0.15−0.83]; 65% relative risk reduction). Relative risk reductions were comparable with overall results across ages and among vaccinated patients.
Conclusions: Real-world nirmatrelvir/ritonavir effectiveness against hospitalization during the Omicron era supports EPIC-HR efficacy among high-risk patients. Future research should confirm these early realworld results and address limitations.
Competing Interests All authors are employees of Pfizer Inc and may hold stock or stock options.
Data Sharing Statement Upon request, and subject to review, Pfizer will provide the summary data that support the findings of this study.
References
Allen, Johnson, Haddock, Game Changer: Paxlovid Reduces Hospitalizations and Saves Lives
Andraska, Alabi, Dorsey, Health care disparities during the COVID-19 pandemic, Semin Vasc Surg, doi:10.1053/j.semvascsurg.2021.08.002
Arbel, Sagy, Hoshen, Nirmatrelvir Use and Severe Covid-19 Outcomes during the Omicron Surge, N Engl J Med, doi:10.1056/NEJMoa2204919
Austin, An Introduction to Propensity Score Methods for Reducing the Effects of Confounding in Observational Studies, Multivariate Behav Res, doi:10.1080/00273171.2011.568786
Dal-Re, Becker, Bottieau, Availability of oral antivirals against SARS-CoV-2 infection and the requirement for an ethical prescribing approach, Lancet Infect Dis, doi:10.1016/S1473-3099(22)00119-0
Docherty, Harrison, Green, Features of 20 133 UK patients in hospital with covid-19 using the ISARIC WHO Clinical Characterisation Protocol: prospective observational cohort study, BMJ, doi:10.1136/bmj.m1985
Dryden-Peterson, Kim, Kim, Nirmatrelvir plus ritonavir for early COVID-19 and hospitalization in a large US health system, medRxiv, doi:10.1101/2022.06.14.2227639330US
Griffin, Brennan-Rieder, Ngo, The Importance of Understanding the Stages of COVID-19 in Treatment and Trials, AIDS Rev, doi:10.24875/AIDSRev.200001261
Hammond, Leister-Tebbe, Gardner, Oral Nirmatrelvir for High-Risk, Nonhospitalized Adults with Covid-19, N Engl J Med, doi:10.1056/NEJMoa2118542
Hodcroft, CoVariants: SARS-CoV-2 Mutations and Variants of Interest
Htoo, Measer, Orr, Evaluating Confounding Control in Estimations of Influenza Antiviral Effectiveness in Electronic Health Plan Data, Am J Epidemiol, doi:10.1093/aje/kwac020
Iuliano, Brunkard, Boehmer, Trends in Disease Severity and Health Care Utilization During the Early Omicron Variant Period Compared with Previous SARS-CoV-2 High Transmission Periods -United States, December 2020-January 2022, MMWR Morb Mortal Wkly Rep, doi:10.15585/mmwr.mm7104e4
Jh, Londhe, Brooks, Trends in characteristics and outcomes among US adults hospitalised with COVID-19 throughout 2020: an observational cohort study, BMJ Open, doi:10.1136/bmjopen-2021-055137
Ko, Danielson, Town, Risk Factors for Coronavirus Disease 2019 (COVID-19)-Associated Hospitalization: COVID-19-Associated Hospitalization Surveillance Network and Behavioral Risk Factor Surveillance System, Clin Infect Dis, doi:10.1093/cid/ciaa1419
Levesque, Hanley, Kezouh, Problem of immortal time bias in cohort studies: example using statins for preventing progression of diabetes, BMJ, doi:10.1136/bmj.b5087
Li, Zhong, Wang, Clinical determinants of the severity of COVID-19: A systematic review and meta-analysis, PLoS One, doi:10.1371/journal.pone.0250602
Malden, Hong, Lewin, Hospitalization and Emergency Department Encounters for COVID-19 After Paxlovid Treatment -California, December 2021, MMWR Morb Mortal Wkly Rep, doi:10.15585/mmwr.mm7125e2
Matrajt, Brown, Cohen, Could widespread use of antiviral treatment curb the COVID-19 pandemic? A modeling study, medRxiv, doi:10.1101/2021.11.10.21266139
Mude, Oguoma, Nyanhanda, Racial disparities in COVID-19 pandemic cases, hospitalisations, and deaths: A systematic review and meta-analysis, J Glob Health, doi:10.7189/jogh.11.05015
Myers, Liu, The COVID-19 Pandemic Strikes Again and Again and Again, JAMA Netw Open, doi:10.1001/jamanetworkopen.2022.1760
Najjar-Debbiny, Gronich, Weber, Effectiveness of Paxlovid in Reducing Severe COVID-19 and Mortality in High Risk Patients, Clin Infect Dis, doi:10.1093/cid/ciac443
Norgaard, Ehrenstein, Vandenbroucke, Confounding in observational studies based on large health care databases: problems and potential solutions -a primer for the clinician, Clin Epidemiol, doi:10.2147/CLEP.S129879
Owen, Allerton, Anderson, An oral SARS-CoV-2 M(pro) inhibitor clinical candidate for the treatment of COVID-19, Science, doi:10.1126/science.abl4784
Sherman, Anderson, Pan, Real-World Evidence -What Is It and What Can It Tell Us?, N Engl J Med, doi:10.1056/NEJMsb1609216
Singh, Toussi, Hackman, Innovative Randomized Phase I Study and Dosing Regimen Selection to Accelerate and Inform Pivotal COVID-19 Trial of Nirmatrelvir, Clin Pharmacol Ther, doi:10.1002/cpt.2603
Tai, Shah, Doubeni, The Disproportionate Impact of COVID-19 on Racial and Ethnic Minorities in the United States, Clin Infect Dis, doi:10.1093/cid/ciaa815
Toussi, Neutel, Navarro, Pharmacokinetics of Oral Nirmatrelvir/Ritonavir, a Protease Inhibitor for Treatment of COVID-19, in Subjects With Renal Impairment, Clin Pharmacol Ther, doi:10.1002/cpt.2688
Wong, Au, Lau, .2 wave in Hong Kong: an observational study, doi:10.1101/2022.05.26.22275631
Yang, Ma, Lei, A meta-analysis of the association between obesity and COVID-19, Epidemiol Infect, doi:10.1017/S0950268820003027
Zhang, Kim, Lonjon, Balance diagnostics after propensity score matching, Ann Transl Med, doi:10.21037/atm.2018.12.10
Zhou, Rahme, Abrahamowicz, Survival bias associated with time-to-treatment initiation in drug effectiveness evaluation: a comparison of methods, Am J Epidemiol, doi:10.1093/aje/kwi307
DOI record:
{
"DOI": "10.1101/2022.09.13.22279908",
"URL": "http://dx.doi.org/10.1101/2022.09.13.22279908",
"abstract": "<jats:p>Objectives: The aim of this analysis was to describe nirmatrelvir/ritonavir real-world effectiveness in preventing hospitalization among high-risk US COVID-19 patients during SARS-CoV-2 Omicron predominance.\nDesign: An ongoing population-based cohort study with retrospective and prospective collection of electronic healthcare data in the United States.\nMethods: Data for this analysis were collected from the US Optum de-identified COVID-19 Electronic Health Record (EHR) dataset during December 22, 2021 to June 8, 2022. Key eligibility criteria for inclusion in the database analysis were at least 12-years-old; positive SARS-CoV-2 test, COVID-19 diagnosis, or nirmatrelvir/ritonavir prescription; and high risk of severe COVID-19 based on demographic/clinical characteristics. Potential confounders between groups were balanced using propensity score matching (PSM). Immortal time bias was addressed.\nOutcome measures: Hospitalization rates within 30 (primary analysis) or 15 (sensitivity analysis) days from COVID-19 diagnosis overall and within subgroups were evaluated.\nResults: Before PSM, the nirmatrelvir/ritonavir group (n=2811) was less racially diverse, older, and had higher COVID-19 vaccination rates and a greater number of comorbidities than the non-nirmatrelvir/ritonavir group (n=194,542). Baseline characteristics were well balanced across groups (n=2808 and n=10,849, respectively) after PSM. Incidence of hospitalization (95% CI) within 30 days was 1.21% (0.84%, 1.69%) for the nirmatrelvir/ritonavir group and 6.94% (6.03%, 7.94%) for the non-nirmatrelvir/ritonavir group, with a hazard ratio (95% CI) of 0.16 (0.11, 0.22; 84% relative risk reduction). Incidence within 15 days was 0.78% (0.49%, 1.18%) for the nirmatrelvir/ritonavir group and 6.54% (5.65%, 7.52%) for the non-nirmatrelvir/ritonavir group; hazard ratio 0.11 (0.07, 0.17; 89% relative risk reduction). Nirmatrelvir/ritonavir was effective in African American patients (hazard ratio, 0.35 [0.15, 0.83]; 65% relative risk reduction). Relative risk reductions were comparable with overall results across ages and among vaccinated patients.\nConclusions: Real-world nirmatrelvir/ritonavir effectiveness against hospitalization during the Omicron era supports EPIC-HR efficacy among high-risk patients. Future research should confirm these early real-world results and address limitations.</jats:p>",
"accepted": {
"date-parts": [
[
2022,
9,
14
]
]
},
"author": [
{
"ORCID": "http://orcid.org/0000-0002-2109-0012",
"affiliation": [],
"authenticated-orcid": false,
"family": "Zhou",
"given": "Xiaofeng",
"sequence": "first"
},
{
"affiliation": [],
"family": "Kelly",
"given": "Scott P",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Liang",
"given": "Caihua",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Li",
"given": "Ling",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Shen",
"given": "Rongjun",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Leister-Tebbe",
"given": "Heidi",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Terra",
"given": "Steven G",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Gaffney",
"given": "Michael",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Russo",
"given": "Leo J",
"sequence": "additional"
}
],
"container-title": [],
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2022,
9,
15
]
],
"date-time": "2022-09-15T03:25:22Z",
"timestamp": 1663212322000
},
"deposited": {
"date-parts": [
[
2022,
9,
15
]
],
"date-time": "2022-09-15T03:25:23Z",
"timestamp": 1663212323000
},
"group-title": "Infectious Diseases (except HIV/AIDS)",
"indexed": {
"date-parts": [
[
2022,
9,
15
]
],
"date-time": "2022-09-15T03:44:52Z",
"timestamp": 1663213492888
},
"institution": [
{
"name": "medRxiv"
}
],
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2022,
9,
14
]
]
},
"link": [
{
"URL": "https://syndication.highwire.org/content/doi/10.1101/2022.09.13.22279908",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "246",
"original-title": [],
"posted": {
"date-parts": [
[
2022,
9,
14
]
]
},
"prefix": "10.1101",
"published": {
"date-parts": [
[
2022,
9,
14
]
]
},
"publisher": "Cold Spring Harbor Laboratory",
"reference-count": 0,
"references-count": 0,
"relation": {},
"resource": {
"primary": {
"URL": "http://medrxiv.org/lookup/doi/10.1101/2022.09.13.22279908"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subtitle": [],
"subtype": "preprint",
"title": "Real-World Effectiveness of Nirmatrelvir/Ritonavir in Preventing Hospitalization Among Patients With COVID-19 at High Risk for Severe Disease in the United States: A Nationwide Population-Based Cohort Study",
"type": "posted-content"
}