Association of Antiviral Drugs for the Treatment of COVID-19 With Acute Renal Failure
et al., In Vivo, doi:10.21873/invivo.13637, Jun 2024
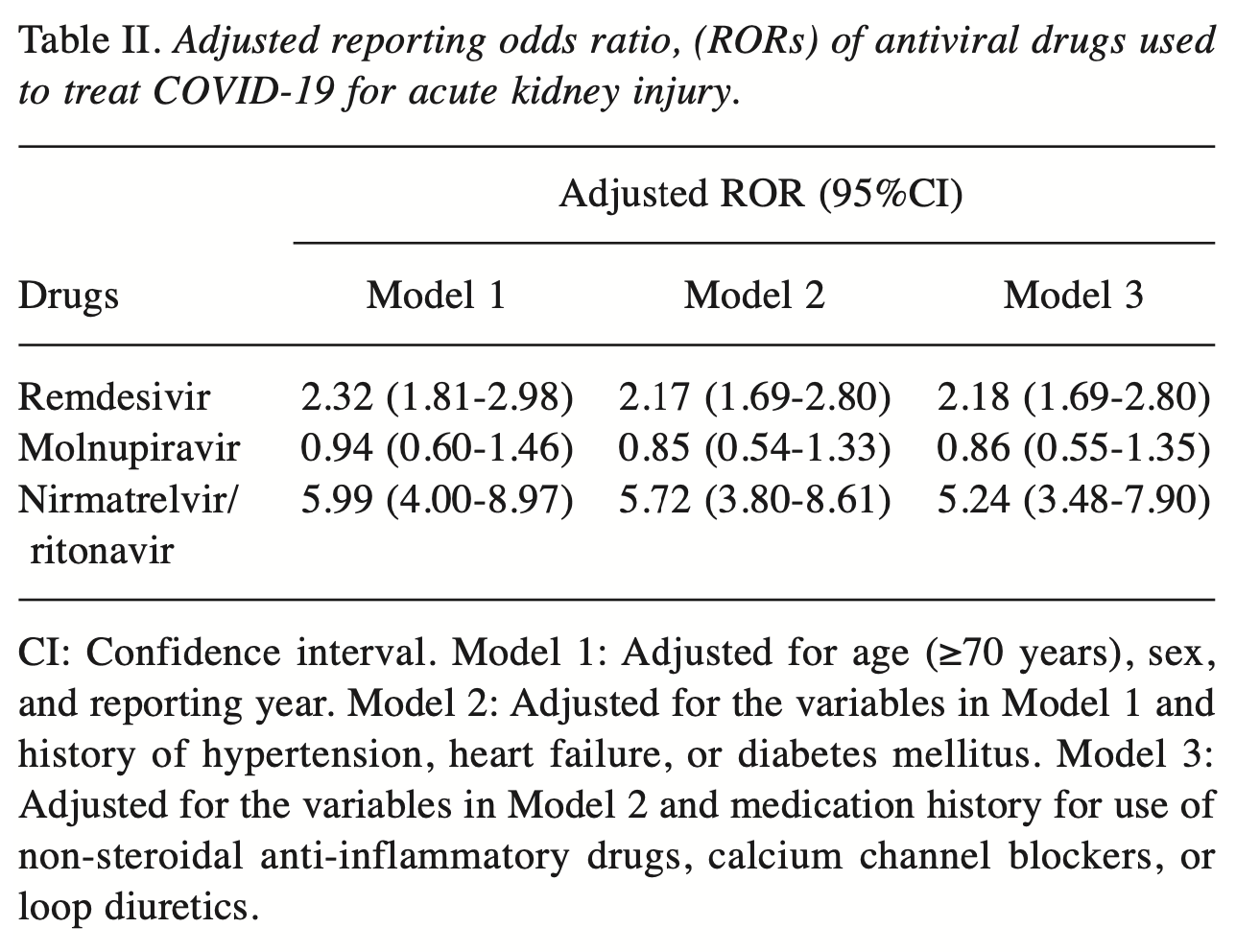
Retrospective 176,197 adverse event reports in Japan showing paxlovid and remdesivir associated with increased risk of acute kidney injury (AKI) in COVID-19 patients.
Study covers paxlovid and remdesivir.
Kamo et al., 27 Jun 2024, Japan, peer-reviewed, 3 authors.
Contact: sogawari@cc.saga-u.ac.jp.
Association of Antiviral Drugs for the Treatment of COVID-19 With Acute Renal Failure
In Vivo, doi:10.21873/invivo.13637
Background/Aim: Reports regarding the association of remdesivir use for the treatment of Coronavirus disease 2019 with the development of acute kidney injury (AKI) are inconsistent, and the associations between the use of other antivirals and AKI remain unclear. Therefore, this study investigated whether the use of antiviral drugs for the treatment of COVID-19 is a risk factor for the development of AKI. Patients and Methods: This study analyzed 176,197 reports submitted to the Japanese Adverse Event Reporting Database between 2020 and 2022. Reporting odds ratios (RORs) and 95% confidence intervals (95%CIs) for AKI that were associated with the use of antiviral drugs in patients with COVID-19 were calculated after adjusting for potential confounders. Results: Overall, 5,879 of the reports analyzed were associated with AKI. Signs of AKI were detected with the use of remdesivir [crude ROR (cROR)=2.45; and nirmatrelvir/ritonavir (cROR=6.07; 95%CI=4.06-9.06). These results were maintained even after adjusting for potential confounders [remdesivir: adjusted ROR (aROR) =2.18; nirmatrelvir/ritonavir: aROR=5.24;]. However, when analyzing data stratified by reporting year, the association between remdesivir and AKI appeared to diminish over time and was not sustained. Conclusion: Nirmatrelvir/ritonavir use may be associated with developing AKI. This knowledge may be useful in helping patients with COVID-19 avoid AKI complications. Coronavirus disease 2019 (COVID-19), caused by Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2), has become a global pandemic. However, on May 4, 2023, the World Health Organization announced the end of its declaration of a "public health emergency of international concern", though mutant strains of COVID-19 continue to emerge and infect individuals. SARS-CoV-2 often causes upper respiratory tract symptoms, including sore throat, nasal discharge, and nasal obstruction, and systemic symptoms, such as fatigue, fever, and myalgia (1). In addition, acute kidney injury (AKI) has been reported as a complication in patients with severe COVID-19 (2). A recent meta-analysis reported that 29% of patients with COVID-19 developed AKI (3). AKI due to COVID-19 is thought to be caused by tubulopenia (4, 5). In addition, antiviral drugs used as therapy against COVID-19 may also be associated with the development of AKI. Remdesivir, molnupiravir, nirmatrelvir/ritonavir, and ensitrelvir are antiviral drugs that are currently used to treat COVID-19. Remdesivir was the first approved treatment, and renal impairment due to accumulation of the additive sulfobutylether-β-cyclodextrin (SBECD) has been reported (6). In addition, an analysis using the Food and Drug Administration Adverse Event Reporting System (FAERS) has shown an association between remdesivir and AKI (7). In contrast, a meta-analysis reported that remdesivir does not affect the risk of AKI (8). Another previous study revealed that remdesivir can be safely used..
Conflicts of Interest The Authors have no conflicts of interest to declare in relation to this study.
Authors' Contributions M.K. and R.S. designed the study and analyzed the data. M.K., R.S. and C.S. drafted the manuscript. All Authors have read and approved the final manuscript.
References
Adamsick, Gandhi, Bidell, Elshaboury, Bhattacharyya et al., Remdesivir in patients with acute or chronic kidney disease and COVID-19, J Am Soc Nephrol, doi:10.1681/ASN.2020050589
Aroca-Martínez, Avendaño-Echavez, Garcia, Ripoll, Dianda et al., Renal tubular dysfunction in COVID-19 patients, Ir J Med Sci, doi:10.1007/s11845-022-02993-0
Capelli, Iacovella, Ghedini, Aiello, Napoletano et al., A case report of tolvaptan therapy for ADPKD patients with COVID-19. The need for appropriate counselling for temporary drug discontinuation, Vivo, doi:10.21873/invivo.12924
Chan, Chaudhary, Saha, Chauhan, Vaid et al., on behalf of the Mount Sinai COVID Informatics Center (MSCIC): AKI in hospitalized patients with COVID-19, J Am Soc Nephrol, doi:10.1681/ASN.2020050615
Chugh, Bird, Alexander, Ritonavir and renal failure, N Engl J Med, doi:10.1056/NEJM199701093360214
Cihlar, Ray, Laflamme, Vela, Tong et al., Molecular assessment of the potential for renal drug interactions between tenofovir and HIV protease inhibitors, Antivir Ther
Deray, Ritonavir-induced acute renal failure, Clin Drug Investig, doi:10.2165/00044011-199816020-00012
Ferlicot, Jamme, Gaillard, Oniszczuk, Couturier et al., Universities/Inserm COVID-19 research collaboration: The spectrum of kidney biopsies in hospitalized patients with COVID-19, acute kidney injury, and/or proteinuria, Nephrol Dial Transplant, doi:10.1093/ndt/gfab042
Goicoechea, Liu, Best, Sun, Jain et al., California Collaborative Treatment Group 578 Team: Greater tenofovir-associated renal function decline with protease inhibitor-based versus nonnucleoside reverse-transcriptase inhibitor-based therapy, J Infect Dis, doi:10.1086/524061
González-Gómez, Teller, González-Barrios, Castro-Frontiñán, Rodríguez-Quesada et al., Safety profile of nirmatrelvir-ritonavir: Evidence of adverse events due to drug-drug interactions, Farm Hosp, doi:10.1016/j.farma.2023.08.005
Hoffman, Dimbil, Erdman, Tatonetti, Overstreet, The Weber effect and the United States Food and Drug Administration's Adverse Event Reporting System (FAERS): analysis of sixty-two drugs approved from 2006 to 2010, Drug Saf, doi:10.1007/s40264-014-0150-2
Ishibashi, Sogawa, Ogata, Matsuoka, Yamada et al., Association between antidiabetic drugs and delirium: a study based on the adverse drug event reporting database in Japan, Clin Drug Investig, doi:10.1007/s40261-023-01337-9
Legrand, Bell, Forni, Joannidis, Koyner et al., Pathophysiology of COVID-19-associated acute kidney injury, Nat Rev Nephrol, doi:10.1038/s41581-021-00452-0
Li, Huang, Wang, Wang, Liang et al., COVID-19 patients' clinical characteristics, discharge rate, and fatality rate of meta-analysis, J Med Virol, doi:10.1002/jmv.25757
Luke, Tomaszewski, Damle, Schlamm, Review of the basic and clinical pharmacology of sulfobutylether-βcydodextrin (SBECD), J Pharm Sci, doi:10.1002/jps.22109
Mocroft, Kirk, Reiss, Wit, Sedlacek et al., EuroSIDA Study Group: Estimated glomerular filtration rate, chronic kidney disease and antiretroviral drug use in HIVpositive patients, AIDS, doi:10.1097/QAD.0b013e328339fe53
Movahed, Akhavizadegan, Dolatkhani, Akbarpour, Nejadghaderi et al., Incidence of acute kidney injury (AKI) and outcomes in COVID-19 patients with and without antiviral medications: A retrospective study, PLoS One, doi:10.1371/journal.pone.0292746
Shams, Kazemi, Jafaryan, Morowvat, Peymani et al., Acute kidney injury in COVID-19 patients receiving remdesivir: A systematic review and meta-analysis of randomized clinical trials, Clinics (Sao Paulo), doi:10.1016/j.clinsp.2023.100200
Sharma, Singh, Drug induced nephrotoxicity-A mechanistic approach, Mol Biol Rep, doi:10.1007/s11033-023-08573-4
Su, Yang, Wan, Yi, Tang et al., Renal histopathological analysis of 26 postmortem findings of patients with COVID-19 in China, Kidney Int, doi:10.1016/j.kint.2020.04.003
Takeuchi, Sogawa, Tsuruhashi, Motooka, Kimura et al., Antihypertensive drug combinations modify cisplatin-induced acute kidney injury, Vivo, doi:10.21873/invivo.12843
Umemura, Mutoh, Mizuno, Hagihara, Kato et al., Safety evaluation of remdesivir for COVID-19 patients with eGFR <30 mL/min without renal replacement therapy in a Japanese single-center study, Healthcare (Basel), doi:10.3390/healthcare10112299
Van Puijenbroek, Bate, Leufkens, Lindquist, Orre et al., A comparison of measures of disproportionality for signal detection in spontaneous reporting systems for adverse drug reactions, Pharmacoepidemiol Drug Saf, doi:10.1002/pds.668
Werion, Belkhir, Perrot, Schmit, Aydin et al., Cliniques universitaires Saint-Luc (CUSL) COVID-19 Research Group: SARS-CoV-2 causes a specific dysfunction of the kidney proximal tubule, Kidney Int, doi:10.1016/j.kint.2020.07.019
Wu, Luo, Wu, He, Li et al., Acute kidney injury associated with remdesivir: a comprehensive pharmacovigilance analysis of COVID-19 reports in FAERS, Front Pharmacol, doi:10.3389/fphar.2022.692828
Yasu, Konuma, Kuroda, Takahashi, Tojo, Effect of cumulative intravenous voriconazole dose on renal function in hematological patients, Antimicrob Agents Chemother, doi:10.1128/AAC.00507-18
Zhang, Pang, Zhou, Meng, Dong et al., Risk factors for acute kidney injury in COVID-19 patients: an updated systematic review and meta-analysis, Ren Fail, doi:10.1080/0886022X.2023.2170809
DOI record:
{
"DOI": "10.21873/invivo.13637",
"ISSN": [
"0258-851X",
"1791-7549"
],
"URL": "http://dx.doi.org/10.21873/invivo.13637",
"accepted": {
"date-parts": [
[
2024,
3,
14
]
]
},
"alternative-id": [
"10.21873/invivo.13637"
],
"author": [
{
"affiliation": [],
"family": "KAMO",
"given": "MASAHIRO",
"sequence": "first"
},
{
"affiliation": [],
"family": "SOGAWA",
"given": "RINTARO",
"sequence": "additional"
},
{
"affiliation": [],
"family": "SHIMANOE",
"given": "CHISATO",
"sequence": "additional"
}
],
"container-title": "In Vivo",
"container-title-short": "In Vivo",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2024,
6,
27
]
],
"date-time": "2024-06-27T14:45:31Z",
"timestamp": 1719499531000
},
"deposited": {
"date-parts": [
[
2024,
7,
1
]
],
"date-time": "2024-07-01T11:35:50Z",
"timestamp": 1719833750000
},
"indexed": {
"date-parts": [
[
2024,
7,
2
]
],
"date-time": "2024-07-02T00:20:08Z",
"timestamp": 1719879608438
},
"is-referenced-by-count": 0,
"issue": "4",
"issued": {
"date-parts": [
[
2024
]
]
},
"journal-issue": {
"issue": "4",
"published-online": {
"date-parts": [
[
2024,
6,
27
]
]
},
"published-print": {
"date-parts": [
[
2024
]
]
}
},
"language": "en",
"link": [
{
"URL": "https://syndication.highwire.org/content/doi/10.21873/invivo.13637",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "9336",
"original-title": [],
"page": "1841-1846",
"prefix": "10.21873",
"published": {
"date-parts": [
[
2024
]
]
},
"published-online": {
"date-parts": [
[
2024,
6,
27
]
]
},
"published-print": {
"date-parts": [
[
2024
]
]
},
"publisher": "Anticancer Research USA Inc.",
"reference-count": 0,
"references-count": 0,
"relation": {},
"resource": {
"primary": {
"URL": "http://iv.iiarjournals.org/lookup/doi/10.21873/invivo.13637"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Association of Antiviral Drugs for the Treatment of COVID-19 With Acute Renal Failure",
"type": "journal-article",
"volume": "38"
}