Acute Kidney Injury Associated With Remdesivir: A Comprehensive Pharmacovigilance Analysis of COVID-19 Reports in FAERS
et al., Frontiers in Pharmacology, doi:10.3389/fphar.2022.692828, Mar 2022
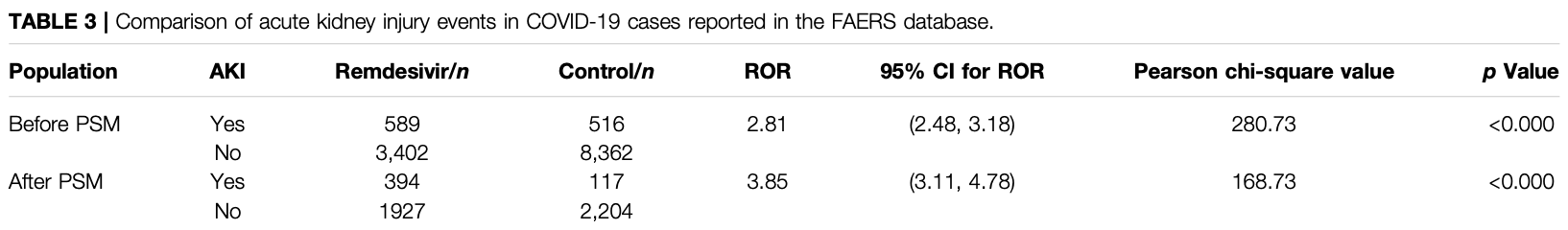
FAERS disproportionality analysis identifying a significant association between remdesivir and AKI, PSM ROR 3.85 [3.11-4.78].
Wu et al., 25 Mar 2022, peer-reviewed, 6 authors.
Contact: clinicpharm_wch@163.com.
Acute Kidney Injury Associated With Remdesivir: A Comprehensive Pharmacovigilance Analysis of COVID-19 Reports in FAERS
Frontiers in Pharmacology, doi:10.3389/fphar.2022.692828
Acute kidney injury (AKI) is a common complication among patients with the novel coronavirus . COVID-19 along with AKI usually resulted in a poor prognosis for those affected. Remdesivir is a novel antiviral drug that was urgently approved for the treatment of COVID-19. In the current study, safety data of remdesivir were limited. We gathered information on COVID-19 cases in patients with adverse events that were reported to the U.S. Food and Drug Administration (US FDA) Adverse Event Reporting System (FAERS) database. We employed the reporting odds ratio (ROR) method to perform disproportionality analysis. Finally, we identified 12,869 COVID-19 cases. A total of 3,991 of these cases reported remdesivir as a primary suspected drug, while 8,878 cases were treated with other drugs. More AKI events occurred in cases of male patients and those above the age of 65 years. We detected a significant association between remdesivir and AKI: ROR = 2.81, 95% CI (2.48, 3.18). The association was stronger after the propensity score matching ROR = 3.85, 95% CI (3.11, 4.78). The mean time to AKI event onset was 4.91 ± 7.25 days in COVID-19 cases with remdesivir therapy. The fatality proportion was 36.45% in AKI cases with remdesivir treatment. This pharmacovigilance study identified a significant association between AKI events and remdesivir treatment in COVID-19 patients by mining FAERS real-world big data. Although causality was not confirmed, the association between remdesivir and AKI should not be ignored, especially in the older, male COVID-19 inpatients.
ETHICS STATEMENT Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. The ethics committee waived the requirement of written informed consent for participation.
AUTHOR CONTRIBUTIONS BW, ML, and TX designed the study; BW, ML, and FW performed the data analysis; BW, ZH, YL, and TX managed and checked all the data; and BW, ML, FW, ZH, and YL wrote the manuscript. All authors read, checked, and approved the final manuscript. Conflict of Interest: The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. Publisher's Note: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Adamsick, Gandhi, Bidell, Elshaboury, Bhattacharyya et al., Remdesivir in Patients with Acute or Chronic Kidney Disease and COVID-19, J. Am. Soc. Nephrol, doi:10.1681/asn.2020050589
Administration, Remdesivir (VEKLURY) drug lable
Alwani, Yassin, Al-Zoubi, Aboumarzouk, Nettleship et al., Sex-based Differences in Severity and Mortality in COVID-19, Rev. Med. Virol, doi:10.1002/rmv.222310.1002/rmv.2223
Armaly, Kinaneh, Skorecki, Renal Manifestations of Covid-19: Physiology and Pathophysiology, J. Clin. Med, doi:10.3390/jcm1006121610.3390/jcm10061216
Bansal, Mahapure, Bhurwal, Gupta, Hassanain et al., Mortality Benefit of Remdesivir in COVID-19: A Systematic Review and Meta-Analysis, Front. Med, doi:10.3389/fmed.2020.606429
Beigel, Tomashek, Dodd, Mehta, Zingman et al., Remdesivir for the Treatment of Covid-19 -Final Report, N. Engl. J. Med, doi:10.1056/NEJMoa2007764
Chan, Chaudhary, Saha, Chauhan, Vaid et al., AKI in Hospitalized Patients with COVID-19, J. Am. Soc. Nephrol, doi:10.1681/asn.2020050615
Chouchana, Preta, Tisseyre, Terrier, Treluyer et al., Kidney Disorders as Serious Adverse Drug Reactions of Remdesivir in Coronavirus Disease 2019: a Retrospective Case-Noncase Study, Kidney Int, doi:10.1016/j.kint.2021.1002.1015
Diebold, Schaub, Landmann, Steiger, Dickenmann, Acute Kidney Injury in Patients with COVID-19: a Retrospective Cohort Study from Switzerland, Swiss Med. Wkly, doi:10.4414/smw.2021.20482
Goldman, Bomze, Dankner, Hod, Meirson et al., Cardiovascular Adverse Events Associated with Hydroxychloroquine and Chloroquine: A Comprehensive Pharmacovigilance Analysis of Pre-COVID-19
Grein, Ohmagari, Shin, Diaz, Asperges et al., Compassionate Use of Remdesivir for Patients with Severe Covid-19, N. Engl. J. Med, doi:10.1056/NEJMoa2007016
Guan, Ni, Hu, Liang, Ou et al., Clinical Characteristics of Coronavirus Disease 2019 in China, N. Engl. J. Med, doi:10.1056/NEJMoa2002032
Gérard, Laurain, Fresse, Parassol, Muzzone et al., Remdesivir and Acute Renal Failure: A Potential Safety Signal from Disproportionality Analysis of the WHO Safety Database, Clin. Pharmacol. Ther, doi:10.1002/cpt.2145
Hirsch, Ng, Ross, Sharma, Shah et al., Acute Kidney Injury in Patients Hospitalized with COVID-19, Kidney Int, doi:10.1016/j.kint.2020.05.006
Huang, Wang, Li, Ren, Zhao et al., Clinical Features of Patients Infected with 2019 Novel Coronavirus in Wuhan, China, Lancet, doi:10.1016/s0140-6736(20)30183-5
Ich, Standardised Medical Dictionary for Regularly Activities Queries
Johnson, Tomlinson, Hawker, Granton, Feldman, Propensity Score Methods for Bias Reduction in Observational Studies of Treatment Effect, Rheum. Dis. Clin. North. Am, doi:10.1016/j.rdc.2018.01.002
Joyner, Bruno, Klassen, Kunze, Johnson et al., Safety Update: COVID-19 Convalescent Plasma in
Kang, Jung, Age-Related Morbidity and Mortality Among Patients with COVID-19, Infect. Chemother, doi:10.3947/ic.2020.52.2.154
Lim, Liang, Assantachai, Auyeung, Kang et al., COVID-19 and Older People in Asia: Asian Working Group for Sarcopenia Calls to Actions, Geriatr. Gerontol. Int, doi:10.1111/ggi.13939
Matta, Chopra, Arora, Morbidity and Mortality Trends of Covid 19 in Top 10 Countries, Indian J. Tuberc, doi:10.1016/j.ijtb.2020.09.031
Palleria, Leporini, Chimirri, Marrazzo, Sacchetta et al., Limitations and Obstacles of the Spontaneous Adverse Drugs Reactions Reporting: Two "challenging" Case Reports, J. Pharmacol. Pharmacother, doi:10.4103/0976-500x.120955
Pettit, Pisano, Nguyen, Lew, Hazra et al., Remdesivir Use in the Setting of Severe Renal Impairment: A Theoretical Concern or Real Risk?, Clin. Infect. Dis, doi:10.1093/cid/ciaa185110.1093/cid/ciaa1851
Pradhan, Olsson, Sex Differences in Severity and Mortality from COVID-19: Are Males More Vulnerable?, Biol. Sex. Differ, doi:10.1186/s13293-13020-00330-13297
Shahid, Kalayanamitra, Mcclafferty, Kepko, Ramgobin et al., COVID-19 and Older Adults: What We Know, J. Am. Geriatr. Soc, doi:10.1111/jgs.16472
Sharma, Ng, Bijol, Jhaveri, Wanchoo, Pathology of COVID-19-Associated Acute Kidney Injury, Clin. Kidney J, doi:10.1093/ckj/sfab003
Su, Yang, Wan, Yi, Tang et al., Renal Histopathological Analysis of 26 Postmortem Findings of Patients with COVID-19 in China, Kidney Int, doi:10.1016/j.kint.2020.04.003
Van Puijenbroek, Bate, Leufkens, Lindquist, Orre et al., A Comparison of Measures of Disproportionality for Signal Detection in Spontaneous Reporting Systems for Adverse Drug Reactions, Pharmacoepidemiol. Drug Saf, doi:10.1002/pds.668
Wang, Zhang, Du, Du, Zhao et al., Remdesivir in Adults with Severe COVID-19: a Randomised, Double-Blind, Placebo-Controlled, Multicentre Trial, Lancet, doi:10.1016/s0140-6736(20)31022-9
Wu, Mcgoogan, Characteristics of and Important Lessons from the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72 314 Cases from the Chinese Center for Disease Control and Prevention, JAMA, doi:10.1001/jama.2020.2648
Yang, Yu, Xu, Shu, Xia et al., Clinical Course and Outcomes of Critically Ill Patients with SARS-CoV-2 Pneumonia in Wuhan, China: a Single-Centered, Retrospective, Observational Study, Lancet Respir. Med, doi:10.1016/s2213-2600(20)30079-5
Yao, Wang, Speicher, Hwang, Cheng et al., Reporting and Guidelines in Propensity Score Analysis: A Systematic Review of Cancer and Cancer Surgical Studies, J. Natl. Cancer Inst, doi:10.1093/jnci/djw323
Zheng, Zhao, Yang, Acute Kidney Injury in COVID-19: The Chinese Experience, Semin. Nephrol, doi:10.1016/j.semnephrol.2020.09.001
DOI record:
{
"DOI": "10.3389/fphar.2022.692828",
"ISSN": [
"1663-9812"
],
"URL": "http://dx.doi.org/10.3389/fphar.2022.692828",
"abstract": "<jats:p>Acute kidney injury (AKI) is a common complication among patients with the novel coronavirus (COVID-19). COVID-19 along with AKI usually resulted in a poor prognosis for those affected. Remdesivir is a novel antiviral drug that was urgently approved for the treatment of COVID-19. In the current study, safety data of remdesivir were limited. We gathered information on COVID-19 cases in patients with adverse events that were reported to the U.S. Food and Drug Administration (US FDA) Adverse Event Reporting System (FAERS) database. We employed the reporting odds ratio (ROR) method to perform disproportionality analysis. Finally, we identified 12,869 COVID-19 cases. A total of 3,991 of these cases reported remdesivir as a primary suspected drug, while 8,878 cases were treated with other drugs. More AKI events occurred in cases of male patients and those above the age of 65 years. We detected a significant association between remdesivir and AKI: ROR = 2.81, 95% CI (2.48, 3.18). The association was stronger after the propensity score matching ROR = 3.85, 95% CI (3.11, 4.78). The mean time to AKI event onset was 4.91 ± 7.25 days in COVID-19 cases with remdesivir therapy. The fatality proportion was 36.45% in AKI cases with remdesivir treatment. This pharmacovigilance study identified a significant association between AKI events and remdesivir treatment in COVID-19 patients by mining FAERS real-world big data. Although causality was not confirmed, the association between remdesivir and AKI should not be ignored, especially in the older, male COVID-19 inpatients.</jats:p>",
"alternative-id": [
"10.3389/fphar.2022.692828"
],
"author": [
{
"affiliation": [],
"family": "Wu",
"given": "Bin",
"sequence": "first"
},
{
"affiliation": [],
"family": "Luo",
"given": "Min",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Wu",
"given": "Fengbo",
"sequence": "additional"
},
{
"affiliation": [],
"family": "He",
"given": "Zhiyao",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Li",
"given": "Yuwen",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Xu",
"given": "Ting",
"sequence": "additional"
}
],
"container-title": "Frontiers in Pharmacology",
"container-title-short": "Front. Pharmacol.",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"frontiersin.org"
]
},
"created": {
"date-parts": [
[
2022,
3,
29
]
],
"date-time": "2022-03-29T18:10:13Z",
"timestamp": 1648577413000
},
"deposited": {
"date-parts": [
[
2022,
3,
29
]
],
"date-time": "2022-03-29T18:10:19Z",
"timestamp": 1648577419000
},
"funder": [
{
"DOI": "10.13039/501100012166",
"award": [
"2020YFC2008302"
],
"doi-asserted-by": "publisher",
"name": "National Key Research and Development Program of China"
}
],
"indexed": {
"date-parts": [
[
2023,
3,
24
]
],
"date-time": "2023-03-24T18:29:47Z",
"timestamp": 1679682587113
},
"is-referenced-by-count": 11,
"issued": {
"date-parts": [
[
2022,
3,
25
]
]
},
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
3,
25
]
],
"date-time": "2022-03-25T00:00:00Z",
"timestamp": 1648166400000
}
}
],
"link": [
{
"URL": "https://www.frontiersin.org/articles/10.3389/fphar.2022.692828/full",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "1965",
"original-title": [],
"prefix": "10.3389",
"published": {
"date-parts": [
[
2022,
3,
25
]
]
},
"published-online": {
"date-parts": [
[
2022,
3,
25
]
]
},
"publisher": "Frontiers Media SA",
"reference": [
{
"DOI": "10.1681/asn.2020050589",
"article-title": "Remdesivir in Patients with Acute or Chronic Kidney Disease and COVID-19",
"author": "Adamsick",
"doi-asserted-by": "publisher",
"first-page": "1384",
"journal-title": "J. Am. Soc. Nephrol.",
"key": "B1",
"volume": "31",
"year": "2020"
},
{
"key": "B2",
"unstructured": "Remdesivir (VEKLURY) drug lable [Online]\n AdministrationF. a. D.\n 2020"
},
{
"DOI": "10.1002/rmv.222310.1002/rmv.2223",
"article-title": "Sex‐based Differences in Severity and Mortality in COVID‐19",
"author": "Alwani",
"doi-asserted-by": "publisher",
"journal-title": "Rev. Med. Virol.",
"key": "B3",
"volume": "31",
"year": "2021"
},
{
"DOI": "10.3390/jcm1006121610.3390/jcm10061216",
"article-title": "Renal Manifestations of Covid-19: Physiology and Pathophysiology",
"author": "Armaly",
"doi-asserted-by": "publisher",
"journal-title": "J. Clin. Med.",
"key": "B4",
"volume": "10",
"year": "2021"
},
{
"DOI": "10.3389/fmed.2020.606429",
"article-title": "Mortality Benefit of Remdesivir in COVID-19: A Systematic Review and Meta-Analysis",
"author": "Bansal",
"doi-asserted-by": "publisher",
"first-page": "606429",
"journal-title": "Front. Med.",
"key": "B5",
"volume": "7",
"year": "2020"
},
{
"DOI": "10.1056/NEJMoa2007764",
"article-title": "Remdesivir for the Treatment of Covid-19 - Final Report",
"author": "Beigel",
"doi-asserted-by": "publisher",
"first-page": "1813",
"journal-title": "N. Engl. J. Med.",
"key": "B6",
"volume": "383",
"year": "2020"
},
{
"DOI": "10.1681/asn.2020050615",
"article-title": "AKI in Hospitalized Patients with COVID-19",
"author": "Chan",
"doi-asserted-by": "publisher",
"first-page": "151",
"journal-title": "J. Am. Soc. Nephrol.",
"key": "B7",
"volume": "32",
"year": "2021"
},
{
"DOI": "10.1016/j.kint.2021.1002.1015",
"article-title": "Kidney Disorders as Serious Adverse Drug Reactions of Remdesivir in Coronavirus Disease 2019: a Retrospective Case-Noncase Study",
"author": "Chouchana",
"doi-asserted-by": "publisher",
"journal-title": "Kidney Int.",
"key": "B8",
"year": "2021"
},
{
"DOI": "10.4414/smw.2021.20482",
"article-title": "Acute Kidney Injury in Patients with COVID-19: a Retrospective Cohort Study from Switzerland",
"author": "Diebold",
"doi-asserted-by": "publisher",
"first-page": "w20482",
"journal-title": "Swiss Med. Wkly",
"key": "B9",
"volume": "151",
"year": "2021"
},
{
"key": "B10",
"unstructured": "Data mining at FDA: white paper [Online]2018"
},
{
"DOI": "10.1002/cpt.2145",
"article-title": "Remdesivir and Acute Renal Failure: A Potential Safety Signal from Disproportionality Analysis of the WHO Safety Database",
"author": "Gérard",
"doi-asserted-by": "publisher",
"first-page": "1021",
"journal-title": "Clin. Pharmacol. Ther.",
"key": "B11",
"volume": "109",
"year": "2021"
},
{
"DOI": "10.1111/bcp.14546",
"article-title": "Cardiovascular Adverse Events Associated with Hydroxychloroquine and Chloroquine: A Comprehensive Pharmacovigilance Analysis of Pre-COVID-19 Reports",
"author": "Goldman",
"doi-asserted-by": "publisher",
"first-page": "1432",
"journal-title": "Br. J. Clin. Pharmacol.",
"key": "B12",
"volume": "87",
"year": "2021"
},
{
"DOI": "10.1056/NEJMoa2007016",
"article-title": "Compassionate Use of Remdesivir for Patients with Severe Covid-19",
"author": "Grein",
"doi-asserted-by": "publisher",
"first-page": "2327",
"journal-title": "N. Engl. J. Med.",
"key": "B13",
"volume": "382",
"year": "2020"
},
{
"DOI": "10.1056/NEJMoa2002032",
"article-title": "Clinical Characteristics of Coronavirus Disease 2019 in China",
"author": "Guan",
"doi-asserted-by": "publisher",
"first-page": "1708",
"journal-title": "N. Engl. J. Med.",
"key": "B14",
"volume": "382",
"year": "2020"
},
{
"DOI": "10.1016/j.kint.2020.05.006",
"article-title": "Acute Kidney Injury in Patients Hospitalized with COVID-19",
"author": "Hirsch",
"doi-asserted-by": "publisher",
"first-page": "209",
"journal-title": "Kidney Int.",
"key": "B15",
"volume": "98",
"year": "2020"
},
{
"DOI": "10.1016/s0140-6736(20)30183-5",
"article-title": "Clinical Features of Patients Infected with 2019 Novel Coronavirus in Wuhan, China",
"author": "Huang",
"doi-asserted-by": "publisher",
"first-page": "497",
"journal-title": "Lancet",
"key": "B16",
"volume": "395",
"year": "2020"
},
{
"key": "B17",
"unstructured": "Standardised Medical Dictionary for Regularly Activities Queries2021"
},
{
"DOI": "10.1016/j.rdc.2018.01.002",
"article-title": "Propensity Score Methods for Bias Reduction in Observational Studies of Treatment Effect",
"author": "Johnson",
"doi-asserted-by": "publisher",
"first-page": "203",
"journal-title": "Rheum. Dis. Clin. North. Am.",
"key": "B18",
"volume": "44",
"year": "2018"
},
{
"DOI": "10.1016/j.mayocp.2020.06.028",
"article-title": "Safety Update: COVID-19 Convalescent Plasma in 20,000 Hospitalized Patients",
"author": "Joyner",
"doi-asserted-by": "publisher",
"first-page": "1888",
"journal-title": "Mayo Clin. Proc.",
"key": "B19",
"volume": "95",
"year": "2020"
},
{
"DOI": "10.3947/ic.2020.52.2.154",
"article-title": "Age-Related Morbidity and Mortality Among Patients with COVID-19",
"author": "Kang",
"doi-asserted-by": "publisher",
"first-page": "154",
"journal-title": "Infect. Chemother.",
"key": "B20",
"volume": "52",
"year": "2020"
},
{
"DOI": "10.1111/ggi.13939",
"article-title": "COVID-19 and Older People in Asia: Asian Working Group for Sarcopenia Calls to Actions",
"author": "Lim",
"doi-asserted-by": "publisher",
"first-page": "547",
"journal-title": "Geriatr. Gerontol. Int.",
"key": "B21",
"volume": "20",
"year": "2020"
},
{
"DOI": "10.1016/j.ijtb.2020.09.031",
"article-title": "Morbidity and Mortality Trends of Covid 19 in Top 10 Countries",
"author": "Matta",
"doi-asserted-by": "publisher",
"first-page": "S167",
"journal-title": "Indian J. Tuberc.",
"key": "B22",
"volume": "67",
"year": "2020"
},
{
"key": "B23",
"volume-title": "Coronavirus Disease 2019 (COVID-19) Treatment Guidelines",
"year": "2021"
},
{
"DOI": "10.4103/0976-500x.120955",
"article-title": "Limitations and Obstacles of the Spontaneous Adverse Drugs Reactions Reporting: Two \"challenging\" Case Reports",
"author": "Palleria",
"doi-asserted-by": "publisher",
"first-page": "S66",
"journal-title": "J. Pharmacol. Pharmacother.",
"key": "B24",
"volume": "4",
"year": "2013"
},
{
"DOI": "10.1093/cid/ciaa185110.1093/cid/ciaa1851",
"article-title": "Remdesivir Use in the Setting of Severe Renal Impairment: A Theoretical Concern or Real Risk?",
"author": "Pettit",
"doi-asserted-by": "publisher",
"first-page": "e3990",
"journal-title": "Clin. Infect. Dis.",
"key": "B25",
"volume": "73",
"year": "2020"
},
{
"DOI": "10.1186/s13293-13020-00330-13297",
"article-title": "Sex Differences in Severity and Mortality from COVID-19: Are Males More Vulnerable?",
"author": "Pradhan",
"doi-asserted-by": "publisher",
"first-page": "53",
"journal-title": "Biol. Sex. Differ.",
"key": "B26",
"volume": "11",
"year": "2020"
},
{
"DOI": "10.1111/jgs.16472",
"article-title": "COVID-19 and Older Adults: What We Know",
"author": "Shahid",
"doi-asserted-by": "publisher",
"first-page": "926",
"journal-title": "J. Am. Geriatr. Soc.",
"key": "B27",
"volume": "68",
"year": "2020"
},
{
"DOI": "10.1093/ckj/sfab003",
"article-title": "Pathology of COVID-19-Associated Acute Kidney Injury",
"author": "Sharma",
"doi-asserted-by": "publisher",
"first-page": "i30",
"journal-title": "Clin. Kidney J.",
"key": "B28",
"volume": "14",
"year": "2021"
},
{
"DOI": "10.1016/j.kint.2020.04.003",
"article-title": "Renal Histopathological Analysis of 26 Postmortem Findings of Patients with COVID-19 in China",
"author": "Su",
"doi-asserted-by": "publisher",
"first-page": "219",
"journal-title": "Kidney Int.",
"key": "B29",
"volume": "98",
"year": "2020"
},
{
"DOI": "10.1002/pds.668",
"article-title": "A Comparison of Measures of Disproportionality for Signal Detection in Spontaneous Reporting Systems for Adverse Drug Reactions",
"author": "van Puijenbroek",
"doi-asserted-by": "publisher",
"first-page": "3",
"journal-title": "Pharmacoepidemiol. Drug Saf.",
"key": "B30",
"volume": "11",
"year": "2002"
},
{
"DOI": "10.1016/s0140-6736(20)31022-9",
"article-title": "Remdesivir in Adults with Severe COVID-19: a Randomised, Double-Blind, Placebo-Controlled, Multicentre Trial",
"author": "Wang",
"doi-asserted-by": "publisher",
"first-page": "1569",
"journal-title": "Lancet",
"key": "B31",
"volume": "395",
"year": "2020"
},
{
"DOI": "10.1001/jama.2020.2648",
"article-title": "Characteristics of and Important Lessons from the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72 314 Cases from the Chinese Center for Disease Control and Prevention",
"author": "Wu",
"doi-asserted-by": "publisher",
"first-page": "1239",
"journal-title": "JAMA",
"key": "B32",
"volume": "323",
"year": "2020"
},
{
"DOI": "10.1016/s2213-2600(20)30079-5",
"article-title": "Clinical Course and Outcomes of Critically Ill Patients with SARS-CoV-2 Pneumonia in Wuhan, China: a Single-Centered, Retrospective, Observational Study",
"author": "Yang",
"doi-asserted-by": "publisher",
"first-page": "475",
"journal-title": "Lancet Respir. Med.",
"key": "B33",
"volume": "8",
"year": "2020"
},
{
"DOI": "10.1093/jnci/djw323",
"article-title": "Reporting and Guidelines in Propensity Score Analysis: A Systematic Review of Cancer and Cancer Surgical Studies",
"author": "Yao",
"doi-asserted-by": "publisher",
"journal-title": "J. Natl. Cancer Inst.",
"key": "B34",
"volume": "109",
"year": "2017"
},
{
"DOI": "10.1016/j.semnephrol.2020.09.001",
"article-title": "Acute Kidney Injury in COVID-19: The Chinese Experience",
"author": "Zheng",
"doi-asserted-by": "publisher",
"first-page": "430",
"journal-title": "Semin. Nephrol.",
"key": "B35",
"volume": "40",
"year": "2020"
}
],
"reference-count": 35,
"references-count": 35,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.frontiersin.org/articles/10.3389/fphar.2022.692828/full"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Pharmacology (medical)",
"Pharmacology"
],
"subtitle": [],
"title": "Acute Kidney Injury Associated With Remdesivir: A Comprehensive Pharmacovigilance Analysis of COVID-19 Reports in FAERS",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.3389/crossmark-policy",
"volume": "13"
}