Remdesivir and Acute Renal Failure: A Potential Safety Signal From Disproportionality Analysis of the WHO Safety Database
et al., Clinical Pharmacology & Therapeutics, doi:10.1002/cpt.2145, Dec 2020
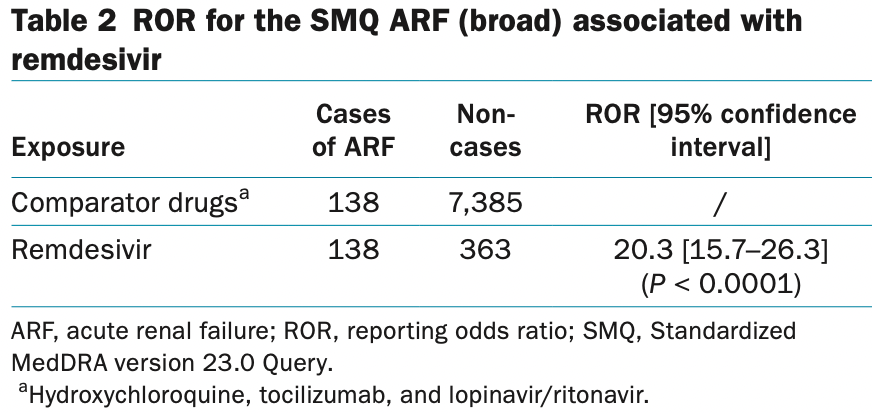
VigiBase retrospective showing a 20x increase in the risk of acute renal failure with remdesivir.
Gérard, Zhou, Wu, Kamo, Choi, Kim show increased risk of acute kidney injury, Leo, Briciu, Muntean, Petrov show increased risk of liver injury, and Negru, Cheng, Mohammed, Kwok show increased risk of cardiac disorders with remdesivir.
1.
Gérard et al., Remdesivir and Acute Renal Failure: A Potential Safety Signal From Disproportionality Analysis of the WHO Safety Database, Clinical Pharmacology & Therapeutics, doi:10.1002/cpt.2145.
2.
Zhou et al., Acute Kidney Injury and Drugs Prescribed for COVID-19 in Diabetes Patients: A Real-World Disproportionality Analysis, Frontiers in Pharmacology, doi:10.3389/fphar.2022.833679.
3.
Wu et al., Acute Kidney Injury Associated With Remdesivir: A Comprehensive Pharmacovigilance Analysis of COVID-19 Reports in FAERS, Frontiers in Pharmacology, doi:10.3389/fphar.2022.692828.
4.
Kamo et al., Association of Antiviral Drugs for the Treatment of COVID-19 With Acute Renal Failure, In Vivo, doi:10.21873/invivo.13637.
5.
Choi et al., Comparative effectiveness of combination therapy with nirmatrelvir–ritonavir and remdesivir versus monotherapy with remdesivir or nirmatrelvir–ritonavir in patients hospitalised with COVID-19: a target trial emulation study, The Lancet Infectious Diseases, doi:10.1016/S1473-3099(24)00353-0.
6.
Kim et al., Investigating the Safety Profile of Fast‐Track COVID‐19 Drugs Using the FDA Adverse Event Reporting System Database: A Comparative Observational Study, Pharmacoepidemiology and Drug Safety, doi:10.1002/pds.70043.
7.
Leo et al., Hepatocellular liver injury in hospitalized patients affected by COVID-19: Presence of different risk factors at different time points, Digestive and Liver Disease, doi:10.1016/j.dld.2021.12.014.
8.
Briciu et al., Evolving Clinical Manifestations and Outcomes in COVID-19 Patients: A Comparative Analysis of SARS-CoV-2 Variant Waves in a Romanian Hospital Setting, Pathogens, doi:10.3390/pathogens12121453.
9.
Muntean et al., Effects of COVID-19 on the Liver and Mortality in Patients with SARS-CoV-2 Pneumonia Caused by Delta and Non-Delta Variants: An Analysis in a Single Centre, Pharmaceuticals, doi:10.3390/ph17010003.
10.
Petrov et al., The Effect of Potentially Hepatotoxic Medicinal Products on Alanine Transaminase Levels in COVID-19 Patients: A Case–Control Study, Safety and Risk of Pharmacotherapy, doi:10.30895/2312-7821-2025-458.
11.
Negru et al., Comparative Pharmacovigilance Analysis of Approved and Repurposed Antivirals for COVID-19: Insights from EudraVigilance Data, Biomedicines, doi:10.3390/biomedicines13061387.
12.
Cheng et al., Cardiovascular Safety of COVID-19 Treatments: A Disproportionality Analysis of Adverse Event Reports from the WHO VigiBase, Infectious Diseases and Therapy, doi:10.1007/s40121-025-01225-z.
Gérard et al., 19 Dec 2020, peer-reviewed, 8 authors.
Abstract: BRIEF REPORT
Remdesivir and Acute Renal Failure: A
Potential Safety Signal From Disproportionality
Analysis of the WHO Safety Database
Alexandre O. Gérard1,2, Audrey Laurain1, Audrey Fresse2, Nadège Parassol2, Marine Muzzone2,
Fanny Rocher2, Vincent L.M. Esnault1 and Milou-Daniel Drici2,*
Remdesivir is approved for emergency use by the US Food and Drug Administration (FDA) and authorized
conditionally by the European Medicines Agency (EMA) for patients with coronavirus disease 2019 (COVID-19).
Its benefit-risk ratio is still being explored because data in the field are rather scant. A decrease of the creatinine
clearance associated with remdesivir has been inconstantly reported in clinical trials with unclear relevance.
Despite these uncertainties, we searched for a potential signal of acute renal failure (ARF) in pharmacovigilance
postmarketing data. An analysis of the international pharmacovigilance postmarketing databases (VigiBase) of
the World Health Organization (WHO) was performed, using two disproportionality methods. Reporting odds ratio
(ROR) compared the number of ARF cases reported with remdesivir, with those reported with other drugs prescribed
in comparable situations of COVID-19 (hydroxychloroquine, tocilizumab, and lopinavir/ritonavir). The combination
of the terms “acute renal failure” and “remdesivir” yielded a statistically significant disproportionality signal with
138 observed cases instead of the 9 expected. ROR of ARF with remdesivir was 20-fold (20.3; confidence interval
0.95 [15.7–26.3], P < 0.0001]) that of comparative drugs. Based on ARF cases reported in VigiBase, and despite
the caveats inherent to COVID-19 circumstances, we detected a statistically significant pharmacovigilance signal
of nephrotoxicity associated with remdesivir, deserving a thorough qualitative assessment of all available data.
Meanwhile, as recommended in its Summary of Product Characteristics, assessment of patients with COVID-19 renal
function should prevail before and during treatment with remdesivir in COVID-19.
Study Highlights
WHAT IS THE CURRENT KNOWLEDGE ON THE
TOPIC?
Remdesivir is a nucleoside analog recently approved
by the US Food and Drug Administration (FDA) for emergency use and conditionally authorized by the European
Medicines Agency (EMA) in patients with coronavirus
disease 2019 (COVID-19), although data pertaining to
its effectiveness and safety are scant. A decrease of the
creatinine clearance has been reported inconstantly in clinical
trials.
WHAT QUESTION DID THIS STUDY ADDRESS?
Is there a potential signal of a risk of acute renal failure associated with remdesivir in pharmacovigilance postmarketing
databases?
WHAT DOES THIS STUDY ADD TO OUR KNOWLEDGE?
There is a statistically suggestive disproportionality signal
regarding the association of acute renal failure and remdesivir.
This possible signal stood out when calculating the reporting odds ratio compared with 3 drugs used in patients with
COVID-19. However, many confounding factors persist.
HOW MIGHT THIS CHANGE CLINICAL PHARMACOLOGY OR TRANSLATIONAL SCIENCE?
These results raise awareness about a possible pharmacovigilance signal regarding the risk of acute renal failure associated
with remdesivir, currently being qualitatively explored by regulatory agencies. Meanwhile, close serum creatinine monitoring
seems warranted in patients treated with remdesivir.
Remdesivir is an antiviral prodrug belonging to the adenosine
nucleoside analog family, which is..
DOI record:
{
"DOI": "10.1002/cpt.2145",
"ISSN": [
"0009-9236",
"1532-6535"
],
"URL": "http://dx.doi.org/10.1002/cpt.2145",
"alternative-id": [
"10.1002/cpt.2145"
],
"archive": [
"Portico"
],
"assertion": [
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Received",
"name": "received",
"order": 0,
"value": "2020-10-08"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Accepted",
"name": "accepted",
"order": 1,
"value": "2020-12-15"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Published",
"name": "published",
"order": 2,
"value": "2021-01-16"
}
],
"author": [
{
"affiliation": [
{
"name": "Department of Nephrology‐Dialysis‐Transplantation Centre Hospitalier Universitaire de Nice Nice Cedex France"
},
{
"name": "Department of Pharmacology and Pharmacovigilance Centre Hospitalier Universitaire de Nice Nice Cedex France"
}
],
"family": "Gérard",
"given": "Alexandre O.",
"sequence": "first"
},
{
"affiliation": [
{
"name": "Department of Nephrology‐Dialysis‐Transplantation Centre Hospitalier Universitaire de Nice Nice Cedex France"
}
],
"family": "Laurain",
"given": "Audrey",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Pharmacology and Pharmacovigilance Centre Hospitalier Universitaire de Nice Nice Cedex France"
}
],
"family": "Fresse",
"given": "Audrey",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Pharmacology and Pharmacovigilance Centre Hospitalier Universitaire de Nice Nice Cedex France"
}
],
"family": "Parassol",
"given": "Nadège",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Pharmacology and Pharmacovigilance Centre Hospitalier Universitaire de Nice Nice Cedex France"
}
],
"family": "Muzzone",
"given": "Marine",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Pharmacology and Pharmacovigilance Centre Hospitalier Universitaire de Nice Nice Cedex France"
}
],
"family": "Rocher",
"given": "Fanny",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Nephrology‐Dialysis‐Transplantation Centre Hospitalier Universitaire de Nice Nice Cedex France"
}
],
"family": "Esnault",
"given": "Vincent L.M.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Pharmacology and Pharmacovigilance Centre Hospitalier Universitaire de Nice Nice Cedex France"
}
],
"family": "Drici",
"given": "Milou‐Daniel",
"sequence": "additional"
}
],
"container-title": [
"Clinical Pharmacology & Therapeutics"
],
"content-domain": {
"crossmark-restriction": true,
"domain": [
"onlinelibrary.wiley.com"
]
},
"created": {
"date-parts": [
[
2020,
12,
19
]
],
"date-time": "2020-12-19T19:46:41Z",
"timestamp": 1608407201000
},
"deposited": {
"date-parts": [
[
2021,
3,
26
]
],
"date-time": "2021-03-26T11:25:02Z",
"timestamp": 1616757902000
},
"indexed": {
"date-parts": [
[
2022,
3,
15
]
],
"date-time": "2022-03-15T06:51:49Z",
"timestamp": 1647327109555
},
"is-referenced-by-count": 12,
"issn-type": [
{
"type": "print",
"value": "0009-9236"
},
{
"type": "electronic",
"value": "1532-6535"
}
],
"issue": "4",
"issued": {
"date-parts": [
[
2021,
1,
16
]
]
},
"journal-issue": {
"issue": "4",
"published-print": {
"date-parts": [
[
2021,
4
]
]
}
},
"language": "en",
"license": [
{
"URL": "http://onlinelibrary.wiley.com/termsAndConditions#vor",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2021,
1,
16
]
],
"date-time": "2021-01-16T00:00:00Z",
"timestamp": 1610755200000
}
},
{
"URL": "http://doi.wiley.com/10.1002/tdm_license_1.1",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2021,
1,
16
]
],
"date-time": "2021-01-16T00:00:00Z",
"timestamp": 1610755200000
}
}
],
"link": [
{
"URL": "https://onlinelibrary.wiley.com/doi/pdf/10.1002/cpt.2145",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://onlinelibrary.wiley.com/doi/full-xml/10.1002/cpt.2145",
"content-type": "application/xml",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://onlinelibrary.wiley.com/doi/pdf/10.1002/cpt.2145",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "311",
"original-title": [],
"page": "1021-1024",
"prefix": "10.1002",
"published": {
"date-parts": [
[
2021,
1,
16
]
]
},
"published-online": {
"date-parts": [
[
2021,
1,
16
]
]
},
"published-print": {
"date-parts": [
[
2021,
4
]
]
},
"publisher": "Wiley",
"reference": [
{
"DOI": "10.1002/phar.2429",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_1_1"
},
{
"DOI": "10.3390/v11040326",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_2_1"
},
{
"DOI": "10.1128/mBio.00221-18",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_3_1"
},
{
"key": "e_1_2_10_4_1",
"unstructured": "FDA.Coronavirus (COVID‐19) Update: FDA Issues Emergency Use Authorization for Potential COVID‐19 Treatment. FDA <https://www.fda.gov/news‐events/press‐announcements/coronavirus‐covid‐19‐update‐fda‐issues‐emergency‐use‐authorization‐potential‐covid‐19‐treatment> (2020)."
},
{
"key": "e_1_2_10_5_1",
"unstructured": "EMA.First COVID‐19 treatment recommended for EU authorisation. European Medicines Agency <https://www.ema.europa.eu/en/news/first‐covid‐19‐treatment‐recommended‐eu‐authorisation> (2020)."
},
{
"DOI": "10.1136/bmj.m3049",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_6_1"
},
{
"DOI": "10.1016/j.biopha.2020.110532",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_7_1"
},
{
"key": "e_1_2_10_8_1",
"unstructured": "European Medicines Agency.EMA ‐ Summary on compassionate use ‐ Remdesivir<https://www.ema.europa.eu/en/documents/other/summary‐compassionate‐use‐remdesivir‐gilead_en.pdf> (2020)."
},
{
"DOI": "10.1002/jps.22109",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_9_1"
},
{
"DOI": "10.1681/ASN.2020050589",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_10_1"
},
{
"DOI": "10.1681/ASN.2020040509",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_11_1"
},
{
"DOI": "10.1056/NEJMoa2015301",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_12_1"
},
{
"DOI": "10.1016/j.phrs.2020.104899",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_13_1"
},
{
"article-title": "Remdesivir for the treatment of Covid‐19 — preliminary report",
"author": "Beigel J.H.",
"journal-title": "N. Engl. J. Med.",
"key": "e_1_2_10_14_1"
},
{
"DOI": "10.1001/jama.2020.16349",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_15_1"
},
{
"DOI": "10.1177/009286150804200501",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_16_1"
},
{
"DOI": "10.1007/s002280050466",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_17_1"
},
{
"key": "e_1_2_10_18_1",
"unstructured": "Standardised MedDRA Queries | MedDRA<https://www.meddra.org/standardised‐meddra‐queries>."
},
{
"DOI": "10.2165/00002018-200225060-00010",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_19_1"
},
{
"key": "e_1_2_10_20_1",
"unstructured": "EMA.Meeting highlights from the Pharmacovigilance Risk Assessment Committee (PRAC) 28 September ‐ 1 October 2020. European Medicines Agency <https://www.ema.europa.eu/en/news/meeting‐highlights‐pharmacovigilance‐risk‐assessment‐committee‐prac‐28‐september‐1‐october‐2020> (2020)."
},
{
"DOI": "10.1056/NEJMc2011400",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_21_1"
},
{
"DOI": "10.1016/j.cgh.2020.07.050",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_22_1"
},
{
"DOI": "10.1111/jgh.12499",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_23_1"
},
{
"DOI": "10.1053/j.ajkd.2005.02.010",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_24_1"
},
{
"DOI": "10.1002/pds.995",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_25_1"
},
{
"key": "e_1_2_10_26_1",
"unstructured": "WHO Collaborating Centre for International Drug Monitoring.Descriptive analysis of COVID‐19‐related spontaneous reports from VigiBase: interim results<https://www.who.int/medicines/regulation/medicines‐safety/covid‐19‐pv‐report‐10.pdf> (2020)."
}
],
"reference-count": 26,
"references-count": 26,
"relation": {},
"score": 1,
"short-container-title": [
"Clin. Pharmacol. Ther."
],
"short-title": [],
"source": "Crossref",
"subject": [
"Pharmacology (medical)",
"Pharmacology"
],
"subtitle": [],
"title": [
"Remdesivir and Acute Renal Failure: A Potential Safety Signal From Disproportionality Analysis of the WHO Safety Database"
],
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1002/crossmark_policy",
"volume": "109"
}