Acute Kidney Injury and Drugs Prescribed for COVID-19 in Diabetes Patients: A Real-World Disproportionality Analysis
et al., Frontiers in Pharmacology, doi:10.3389/fphar.2022.833679, Mar 2022
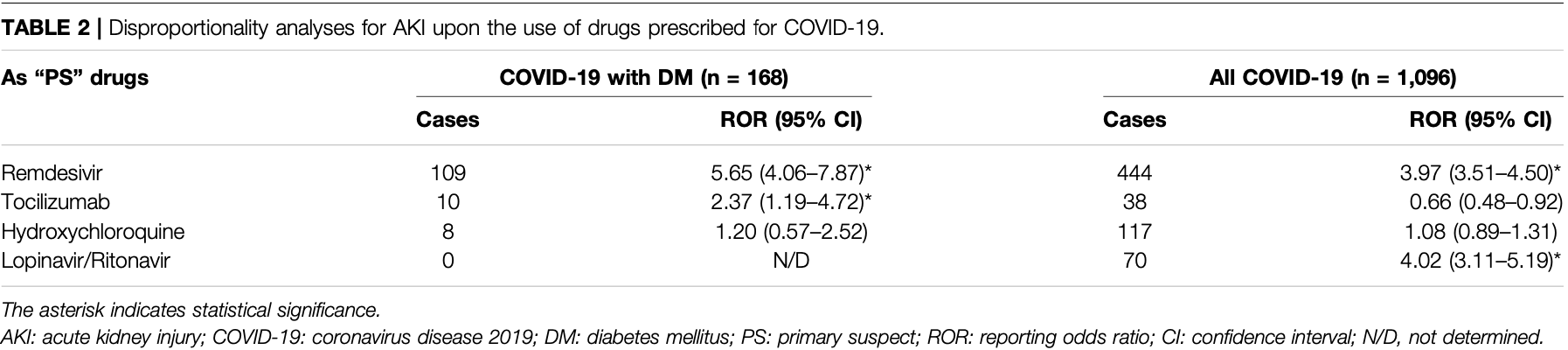
FAERS analysis showing significantly increased risk of acute kidney injury with remdesivir.
Zhou et al., 17 Mar 2022, peer-reviewed, 6 authors.
Acute Kidney Injury and Drugs Prescribed for COVID-19 in Diabetes Patients: A Real-World Disproportionality Analysis
doi:10.3389/fphar.2022.833679
Background: The information is relatively scarce regarding the occurrence of druginduced acute kidney injury (AKI) when anti-coronavirus disease 2019 (COVID-19) drugs are prescribed for patients with diabetes mellitus (DM). Objective: The objective of this study was to evaluate a pharmacovigilance signal for AKI upon the use of common drugs prescribed for COVID-19 treatment, especially in patients with DM.
Methods: The FDA Adverse Event Reporting System (FAERS) database were used, and data from the first quarter of 2020 to the third quarter of 2021 were retrieved. A disproportionality analysis was performed to determine whether AKI was more frequently reported with anti-COVID-19 drugs compared to that with other drugs in different populations. Further, reporting odds ratios (RORs) and their 95% confidence intervals (CIs) were used to calculate disproportionality. Results: We identified 33,488 COVID-19 patients and 2397 COVID-19 patients with DM. AKI was the most frequent adverse drug reaction (ADR) reported in this patient population. The primary suspected drugs related to AKI in more than half of the reports (75.60%, 127/ 168) were four common anti-COVID-19 drugs (remdesivir, tocilizumab, hydroxychloroquine, and lopinavir/ritonavir). Compared with other drugs in the same time window, remdesivir and lopinavir/ritonavir were associated with an increased risk of AKI in all COVID-19 patients (ROR: 3.97, 95% CI: 3.51-4.50; ROR: 4.02, 95% CI: 3.11-5.19, respectively). In COVID-19 patients with DM, remdesivir was significantly associated with AKI (ROR: 5.65, 95% CI: 4.06-7.87); meanwhile, there was a new AKI signal associated with tocilizumab (ROR: 2.37, 95% CI: 1.19-4.72). After sensitivity analyses in COVID-19 patients with DM, consistent results for remdesivir were observed; however, the AKI signals for tocilizumab were unstable.
Conclusion: Our study confirmed the association of AKI with the usage of common anti-COVID-19 drugs (especially remdesivir and tocilizumab) in DM patients. These safety signals suggested more individualized treatments for COVID-19 patients with
ETHICS STATEMENT Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. Written informed consent from the participants' legal guardian/next of kin was not required to participate in this study in accordance with the national legislation and the institutional requirements.
AUTHOR CONTRIBUTIONS YZ, MZ, and JZ were responsible for study design, data acquisition and interpretation, statistical analysis, and writing and editing of the manuscript. JL, LW, and XZ aided in data acquisition and interpretation, statistical analysis. Conflict of Interest: The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. Publisher's Note: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher. Copyright © 2022 Zhou, Li, Wang, Zhu, Zhang and Zheng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited..
References
Akhtar, Benter, Danjuma, Doi, Hasan et al., Pharmacotherapy in COVID-19 Patients: A Review of ACE2-Raising Drugs and Their Clinical Safety, J. Drug Target, doi:10.1080/1061186x.2020.1797754
Binois, Hachad, Salem, Charpentier, Lebrun-Vignes et al., Acute Kidney Injury Associated with Lopinavir/Ritonavir Combined Therapy in Patients with COVID-19, Kidney Int. Rep, doi:10.1016/j.ekir.2020.07.035
Cariou, Hadjadj, Wargny, Pichelin, Al-Salameh et al., Phenotypic Characteristics and Prognosis of Inpatients with COVID-19 and Diabetes: The Coronado Study, Diabetologia, doi:10.1007/s00125-020-05180-x
Chouchana, Preta, Tisseyre, Terrier, Treluyer et al., Kidney Disorders as Serious Adverse Drug Reactions of Remdesivir in Coronavirus Disease 2019: A Retrospective Case-Noncase Study, Kidney Int, doi:10.1016/j.kint.2021.02.015
Drucker, Coronavirus Infections and Type 2 Diabetes-Shared Pathways with Therapeutic Implications, Endocr. Rev, doi:10.1210/endrev/bnaa011
Dubert, Visseaux, Isernia, Bouadma, Deconinck et al., Case Report Study of the First Five COVID-19 Patients Treated with Remdesivir in France, Int. J. Infect. Dis, doi:10.1016/j.ijid.2020.06.093
Gérard, Laurain, Fresse, Parassol, Muzzone et al., Remdesivir and Acute Renal Failure: A Potential Safety Signal from Disproportionality Analysis of the WHO Safety Database, Clin. Pharmacol. Ther, doi:10.1002/cpt.2145
Huang, Lim, Pranata, Diabetes Mellitus Is Associated with Increased Mortality and Severity of Disease in COVID-19 Pneumonia -A Systematic Review, Meta-Analysis, and Meta-Regression, Diabetes Metab. Syndr, doi:10.1016/j.dsx.2020.04.018
Iacobellis, COVID-19 and Diabetes: Can DPP4 Inhibition Play a Role?, Diabetes Res. Clin. Pract, doi:10.1016/j.diabres.2020.108125
Kwiatkowska, Domański, Dziedziejko, Kajdy, Stefańska et al., The Mechanism of Drug Nephrotoxicity and the Methods for Preventing Kidney Damage, Int. J. Mol. Sci, doi:10.3390/ijms22116109
Lim, Bae, Kwon, Nauck, COVID-19 and Diabetes Mellitus: from Pathophysiology to Clinical Management, Nat. Rev. Endocrinol, doi:10.1038/s41574-020-00435-4
Liu, Yan, Wang, Wang, Fu et al., Drug-Induced Hospital-Acquired Acute Kidney Injury in China: A Multicenter Cross-Sectional Survey, Kidney Dis, doi:10.1159/000510455
Longmore, Miller, Bekkering, Saner, Mifsud et al., Diabetes and Overweight/Obesity Are Independent, Nonadditive Risk Factors for In-Hospital Severity of COVID-19: An International, Multicenter Retrospective Meta-Analysis, Diabetes Care, doi:10.2337/dc20-2676
Montastruc, Sommet, Bagheri, Lapeyre-Mestre, Benefits and Strengths of the Disproportionality Analysis for Identification of Adverse Drug Reactions in a Pharmacovigilance Database, Br. J. Clin. Pharmacol, doi:10.1111/j.1365-2125.2011.04037.x
Ng, Tipih, Makoah, Vermeulen, Goedhals et al., Comorbidities in SARS-CoV-2 Patients: A Systematic Review and Meta-Analysis, mBio, doi:10.1128/mBio.03647-20
Noguchi, Tachi, Teramachi, Detection Algorithms and Attentive Points of Safety Signal Using Spontaneous Reporting Systems as a Clinical Data Source, Brief Bioinform, doi:10.1093/bib/bbab347
Richardson, Hirsch, Narasimhan, Crawford, Mcginn et al., Presenting Characteristics, Comorbidities, and Outcomes Among 5700 Patients Hospitalized with COVID-19 in the New York City Area, JAMA, doi:10.1001/jama.2020.6775
Rocca, Gauffin, Savage, Vidlin, Grundmark, Remdesivir in the COVID-19 Pandemic: An Analysis of Spontaneous Reports in VigiBase during 2020, Drug Saf, doi:10.1007/s40264-021-01091-x
Schneider, Jaenigen, Wagner, Rieg, Hornuss et al., Therapy with Lopinavir/Ritonavir and Hydroxychloroquine Is Associated with Acute Kidney Injury in COVID-19 Patients, PLoS One, doi:10.1371/journal.pone.0249760
Singh, Kamath, Assessment of Adverse Events Associated with Remdesivir Use for Coronavirus Disease 2019 Using Real-World Data, Expert Opin. Drug Saf, doi:10.1080/14740338.2021.1962846
Spinner, Gottlieb, Criner, Arribas López, Cattelan et al., Effect of Remdesivir vs Standard Care on Clinical Status at 11 Days in Patients with Moderate COVID-19: A Randomized Clinical Trial, JAMA, doi:10.1001/jama.2020.16349
Yang, Zheng, Gou, Pu, Chen et al., Prevalence of Comorbidities and its Effects in Patients Infected with SARS-CoV-2: A Systematic Review and Meta-Analysis, Int. J. Infect. Dis, doi:10.1016/j.ijid.2020.03.017
Zheng, Zhao, Yang, Acute Kidney Injury in COVID-19: The Chinese Experience, Semin. Nephrol, doi:10.1016/j.semnephrol.2020.09.001
Zhou, Yu, Du, Fan, Liu et al., Clinical Course and Risk Factors for Mortality of Adult Inpatients with COVID-19 in Wuhan, China: A Retrospective Cohort Study, Lancet, doi:10.1016/s0140-6736(20)30566-3
DOI record:
{
"DOI": "10.3389/fphar.2022.833679",
"ISSN": [
"1663-9812"
],
"URL": "http://dx.doi.org/10.3389/fphar.2022.833679",
"abstract": "<jats:p><jats:bold>Background</jats:bold>: The information is relatively scarce regarding the occurrence of drug-induced acute kidney injury (AKI) when anti-coronavirus disease 2019 (COVID-19) drugs are prescribed for patients with diabetes mellitus (DM).</jats:p><jats:p><jats:bold>Objective</jats:bold>: The objective of this study was to evaluate a pharmacovigilance signal for AKI upon the use of common drugs prescribed for COVID-19 treatment, especially in patients with DM.</jats:p><jats:p><jats:bold>Methods</jats:bold>: The FDA Adverse Event Reporting System (FAERS) database were used, and data from the first quarter of 2020 to the third quarter of 2021 were retrieved. A disproportionality analysis was performed to determine whether AKI was more frequently reported with anti-COVID-19 drugs compared to that with other drugs in different populations. Further, reporting odds ratios (RORs) and their 95% confidence intervals (CIs) were used to calculate disproportionality. <jats:bold>Results:</jats:bold> We identified 33,488 COVID-19 patients and 2397 COVID-19 patients with DM. AKI was the most frequent adverse drug reaction (ADR) reported in this patient population. The primary suspected drugs related to AKI in more than half of the reports (75.60%, 127/168) were four common anti-COVID-19 drugs (remdesivir, tocilizumab, hydroxychloroquine, and lopinavir/ritonavir). Compared with other drugs in the same time window, remdesivir and lopinavir/ritonavir were associated with an increased risk of AKI in all COVID-19 patients (ROR: 3.97, 95% CI: 3.51–4.50; ROR: 4.02, 95% CI: 3.11–5.19, respectively). In COVID-19 patients with DM, remdesivir was significantly associated with AKI (ROR: 5.65, 95% CI: 4.06–7.87); meanwhile, there was a new AKI signal associated with tocilizumab (ROR: 2.37, 95% CI: 1.19–4.72). After sensitivity analyses in COVID-19 patients with DM, consistent results for remdesivir were observed; however, the AKI signals for tocilizumab were unstable.</jats:p><jats:p><jats:bold>Conclusion:</jats:bold> Our study confirmed the association of AKI with the usage of common anti-COVID-19 drugs (especially remdesivir and tocilizumab) in DM patients. These safety signals suggested more individualized treatments for COVID-19 patients with comorbidities. Cross-disciplinary collaborative is needed to improve current strategy of clinical treatment and develop new approaches to management.</jats:p>",
"alternative-id": [
"10.3389/fphar.2022.833679"
],
"author": [
{
"affiliation": [],
"family": "Zhou",
"given": "Yu",
"sequence": "first"
},
{
"affiliation": [],
"family": "Li",
"given": "Jianbin",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Wang",
"given": "Linyao",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Zhu",
"given": "Xinyan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Zhang",
"given": "Meilian",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Zheng",
"given": "Jiaping",
"sequence": "additional"
}
],
"container-title": [
"Frontiers in Pharmacology"
],
"content-domain": {
"crossmark-restriction": true,
"domain": [
"frontiersin.org"
]
},
"created": {
"date-parts": [
[
2022,
3,
17
]
],
"date-time": "2022-03-17T06:18:58Z",
"timestamp": 1647497938000
},
"deposited": {
"date-parts": [
[
2022,
3,
17
]
],
"date-time": "2022-03-17T06:19:00Z",
"timestamp": 1647497940000
},
"indexed": {
"date-parts": [
[
2022,
4,
5
]
],
"date-time": "2022-04-05T07:01:53Z",
"timestamp": 1649142113717
},
"is-referenced-by-count": 0,
"issn-type": [
{
"type": "electronic",
"value": "1663-9812"
}
],
"issued": {
"date-parts": [
[
2022,
3,
17
]
]
},
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
3,
17
]
],
"date-time": "2022-03-17T00:00:00Z",
"timestamp": 1647475200000
}
}
],
"link": [
{
"URL": "https://www.frontiersin.org/articles/10.3389/fphar.2022.833679/full",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "1965",
"original-title": [],
"prefix": "10.3389",
"published": {
"date-parts": [
[
2022,
3,
17
]
]
},
"published-online": {
"date-parts": [
[
2022,
3,
17
]
]
},
"publisher": "Frontiers Media SA",
"reference": [
{
"DOI": "10.1080/1061186x.2020.1797754",
"article-title": "Pharmacotherapy in COVID-19 Patients: A Review of ACE2-Raising Drugs and Their Clinical Safety",
"author": "Akhtar",
"doi-asserted-by": "publisher",
"first-page": "683",
"journal-title": "J. Drug Target.",
"key": "B1",
"volume": "28",
"year": "2020"
},
{
"DOI": "10.1016/j.ekir.2020.07.035",
"article-title": "Acute Kidney Injury Associated with Lopinavir/Ritonavir Combined Therapy in Patients with COVID-19",
"author": "Binois",
"doi-asserted-by": "publisher",
"first-page": "1787",
"journal-title": "Kidney Int. Rep.",
"key": "B2",
"volume": "5",
"year": "2020"
},
{
"DOI": "10.1007/s00125-020-05180-x",
"article-title": "Phenotypic Characteristics and Prognosis of Inpatients with COVID-19 and Diabetes: The Coronado Study",
"author": "Cariou",
"doi-asserted-by": "publisher",
"first-page": "1500",
"journal-title": "Diabetologia",
"key": "B3",
"volume": "63",
"year": "2020"
},
{
"DOI": "10.1016/j.kint.2021.02.015",
"article-title": "Kidney Disorders as Serious Adverse Drug Reactions of Remdesivir in Coronavirus Disease 2019: A Retrospective Case-Noncase Study",
"author": "Chouchana",
"doi-asserted-by": "publisher",
"first-page": "1235",
"journal-title": "Kidney Int.",
"key": "B4",
"volume": "99",
"year": "2021"
},
{
"DOI": "10.1210/endrev/bnaa011",
"article-title": "Coronavirus Infections and Type 2 Diabetes-Shared Pathways with Therapeutic Implications",
"author": "Drucker",
"doi-asserted-by": "publisher",
"first-page": "bnaa011",
"journal-title": "Endocr. Rev.",
"key": "B5",
"volume": "41",
"year": "2020"
},
{
"DOI": "10.1016/j.ijid.2020.06.093",
"article-title": "Case Report Study of the First Five COVID-19 Patients Treated with Remdesivir in France",
"author": "Dubert",
"doi-asserted-by": "publisher",
"first-page": "290",
"journal-title": "Int. J. Infect. Dis.",
"key": "B6",
"volume": "98",
"year": "2020"
},
{
"DOI": "10.1002/cpt.2145",
"article-title": "Remdesivir and Acute Renal Failure: A Potential Safety Signal from Disproportionality Analysis of the WHO Safety Database",
"author": "Gérard",
"doi-asserted-by": "publisher",
"first-page": "1021",
"journal-title": "Clin. Pharmacol. Ther.",
"key": "B7",
"volume": "109",
"year": "2021"
},
{
"DOI": "10.1016/j.dsx.2020.04.018",
"article-title": "Diabetes Mellitus Is Associated with Increased Mortality and Severity of Disease in COVID-19 Pneumonia - A Systematic Review, Meta-Analysis, and Meta-Regression",
"author": "Huang",
"doi-asserted-by": "publisher",
"first-page": "395",
"journal-title": "Diabetes Metab. Syndr.",
"key": "B8",
"volume": "14",
"year": "2020"
},
{
"DOI": "10.1016/j.diabres.2020.108125",
"article-title": "COVID-19 and Diabetes: Can DPP4 Inhibition Play a Role?",
"author": "Iacobellis",
"doi-asserted-by": "publisher",
"first-page": "108125",
"journal-title": "Diabetes Res. Clin. Pract.",
"key": "B9",
"volume": "162",
"year": "2020"
},
{
"DOI": "10.3390/ijms22116109",
"article-title": "The Mechanism of Drug Nephrotoxicity and the Methods for Preventing Kidney Damage",
"author": "Kwiatkowska",
"doi-asserted-by": "publisher",
"first-page": "6109",
"journal-title": "Int. J. Mol. Sci.",
"key": "B10",
"volume": "22",
"year": "2021"
},
{
"DOI": "10.1038/s41574-020-00435-4",
"article-title": "COVID-19 and Diabetes Mellitus: from Pathophysiology to Clinical Management",
"author": "Lim",
"doi-asserted-by": "publisher",
"first-page": "11",
"journal-title": "Nat. Rev. Endocrinol.",
"key": "B11",
"volume": "17",
"year": "2021"
},
{
"DOI": "10.1159/000510455",
"article-title": "Drug-Induced Hospital-Acquired Acute Kidney Injury in China: A Multicenter Cross-Sectional Survey",
"author": "Liu",
"doi-asserted-by": "publisher",
"first-page": "143",
"journal-title": "Kidney Dis. (Basel)",
"key": "B12",
"volume": "7",
"year": "2021"
},
{
"DOI": "10.2337/dc20-2676",
"article-title": "Diabetes and Overweight/Obesity Are Independent, Nonadditive Risk Factors for In-Hospital Severity of COVID-19: An International, Multicenter Retrospective Meta-Analysis",
"author": "Longmore",
"doi-asserted-by": "publisher",
"first-page": "1281",
"journal-title": "Diabetes Care",
"key": "B13",
"volume": "44",
"year": "2021"
},
{
"DOI": "10.1111/j.1365-2125.2011.04037.x",
"article-title": "Benefits and Strengths of the Disproportionality Analysis for Identification of Adverse Drug Reactions in a Pharmacovigilance Database",
"author": "Montastruc",
"doi-asserted-by": "publisher",
"first-page": "905",
"journal-title": "Br. J. Clin. Pharmacol.",
"key": "B14",
"volume": "72",
"year": "2011"
},
{
"DOI": "10.1128/mBio.03647-20",
"article-title": "Comorbidities in SARS-CoV-2 Patients: A Systematic Review and Meta-Analysis",
"author": "Ng",
"doi-asserted-by": "publisher",
"first-page": "e03647",
"journal-title": "mBio",
"key": "B15",
"volume": "12",
"year": "2021"
},
{
"DOI": "10.1093/bib/bbab347",
"article-title": "Detection Algorithms and Attentive Points of Safety Signal Using Spontaneous Reporting Systems as a Clinical Data Source",
"author": "Noguchi",
"doi-asserted-by": "publisher",
"first-page": "bbab347",
"journal-title": "Brief Bioinform",
"key": "B16",
"volume": "22",
"year": "2021"
},
{
"DOI": "10.1001/jama.2020.6775",
"article-title": "Presenting Characteristics, Comorbidities, and Outcomes Among 5700 Patients Hospitalized with COVID-19 in the New York City Area",
"author": "Richardson",
"doi-asserted-by": "publisher",
"first-page": "2052",
"journal-title": "JAMA",
"key": "B17",
"volume": "323",
"year": "2020"
},
{
"DOI": "10.1007/s40264-021-01091-x",
"article-title": "Remdesivir in the COVID-19 Pandemic: An Analysis of Spontaneous Reports in VigiBase during 2020",
"author": "Rocca",
"doi-asserted-by": "publisher",
"first-page": "987",
"journal-title": "Drug Saf.",
"key": "B18",
"volume": "44",
"year": "2021"
},
{
"DOI": "10.1371/journal.pone.0249760",
"article-title": "Therapy with Lopinavir/Ritonavir and Hydroxychloroquine Is Associated with Acute Kidney Injury in COVID-19 Patients",
"author": "Schneider",
"doi-asserted-by": "publisher",
"first-page": "e0249760",
"journal-title": "PLoS One",
"key": "B19",
"volume": "16",
"year": "2021"
},
{
"DOI": "10.1080/14740338.2021.1962846",
"article-title": "Assessment of Adverse Events Associated with Remdesivir Use for Coronavirus Disease 2019 Using Real-World Data",
"author": "Singh",
"doi-asserted-by": "publisher",
"first-page": "1559",
"journal-title": "Expert Opin. Drug Saf.",
"key": "B20",
"volume": "20",
"year": "2021"
},
{
"DOI": "10.1001/jama.2020.16349",
"article-title": "Effect of Remdesivir vs Standard Care on Clinical Status at 11 Days in Patients with Moderate COVID-19: A Randomized Clinical Trial",
"author": "Spinner",
"doi-asserted-by": "publisher",
"first-page": "1048",
"journal-title": "JAMA",
"key": "B21",
"volume": "324",
"year": "2020"
},
{
"DOI": "10.1016/j.ijid.2020.03.017",
"article-title": "Prevalence of Comorbidities and its Effects in Patients Infected with SARS-CoV-2: A Systematic Review and Meta-Analysis",
"author": "Yang",
"doi-asserted-by": "publisher",
"first-page": "91",
"journal-title": "Int. J. Infect. Dis.",
"key": "B22",
"volume": "94",
"year": "2020"
},
{
"DOI": "10.1016/j.semnephrol.2020.09.001",
"article-title": "Acute Kidney Injury in COVID-19: The Chinese Experience",
"author": "Zheng",
"doi-asserted-by": "publisher",
"first-page": "430",
"journal-title": "Semin. Nephrol.",
"key": "B23",
"volume": "40",
"year": "2020"
},
{
"DOI": "10.1016/s0140-6736(20)30566-3",
"article-title": "Clinical Course and Risk Factors for Mortality of Adult Inpatients with COVID-19 in Wuhan, China: A Retrospective Cohort Study",
"author": "Zhou",
"doi-asserted-by": "publisher",
"first-page": "1054",
"journal-title": "Lancet",
"key": "B24",
"volume": "395",
"year": "2020"
}
],
"reference-count": 24,
"references-count": 24,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.frontiersin.org/articles/10.3389/fphar.2022.833679/full"
}
},
"score": 1,
"short-container-title": [
"Front. Pharmacol."
],
"short-title": [],
"source": "Crossref",
"subject": [
"Pharmacology (medical)",
"Pharmacology"
],
"subtitle": [],
"title": [
"Acute Kidney Injury and Drugs Prescribed for COVID-19 in Diabetes Patients: A Real-World Disproportionality Analysis"
],
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.3389/crossmark-policy",
"volume": "13"
}