Feb 9 2024 |
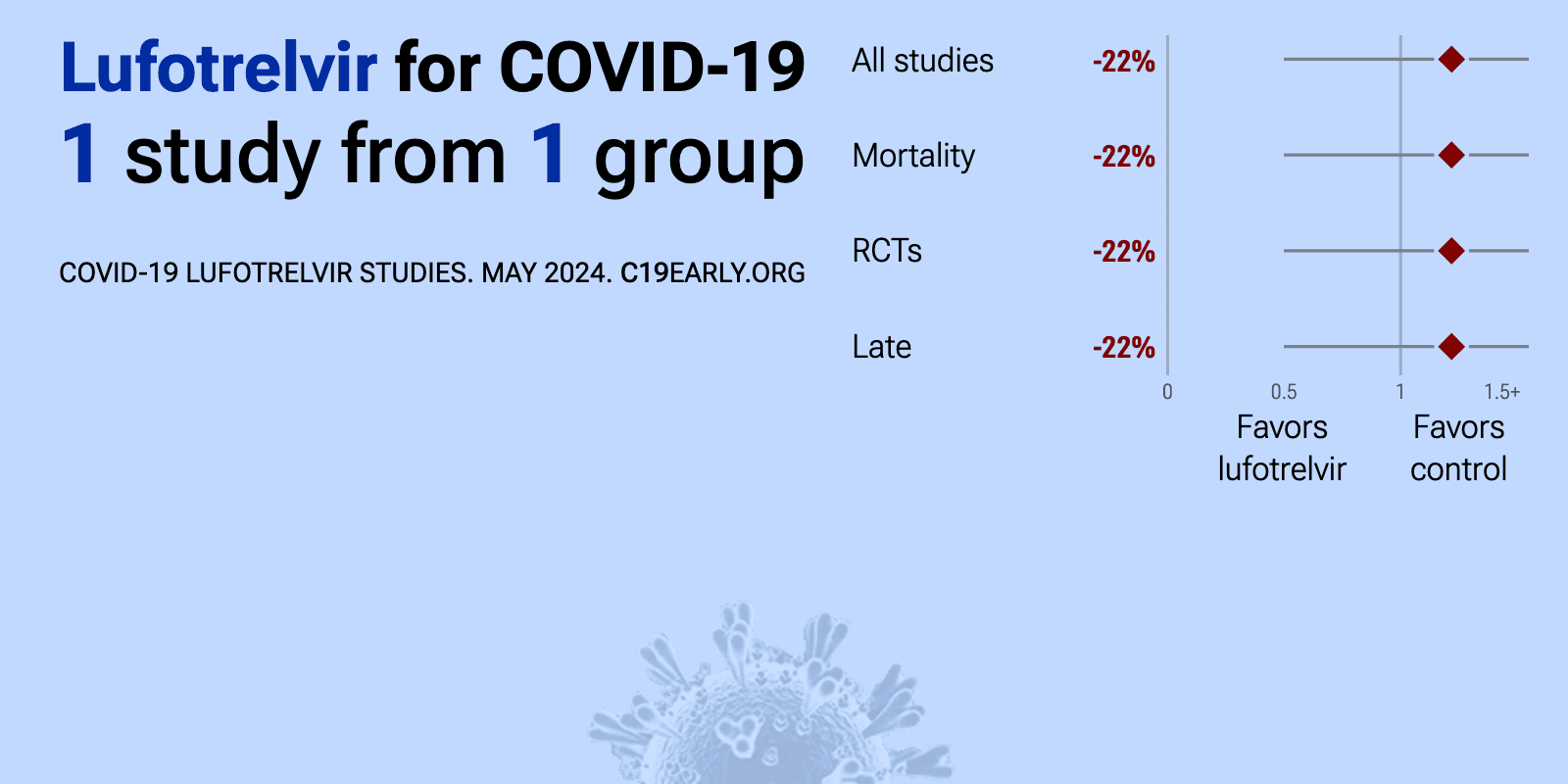
et al., NCT05780541 | PF-07304814 for Inpatients With COVID-19 (An ACTIV-3/TICO Treatment Trial) |
| 22% higher mortality (p=0.77) and 44% higher hospital discharge (p=0.11). RCT 58 patients showing no significant differences with lufotrelvir. This is a 2021 study which has not been published yet. Results were made available on clinicaltrials.gov in 2024. | ||
Jul 10 2023 |
et al., Open Forum Infectious Diseases, doi:10.1093/ofid/ofad355 | Safety, Tolerability, and Pharmacokinetics of Single and Multiple Ascending Intravenous Infusions of PF-07304814 (Lufotrelvir) in Participants Hospitalized With COVID-19 |
| Phase 1 safety and pharmacokinetic study of lufotrelvir in 25 hospitalized COVID-19 patients. No adverse events or serious adverse events were considered related to lufotrelvir. Concentrations of lufotrelvir and its active moiety increase.. | ||
Nov 11 2022 |
et al., Frontiers in Pharmacology, doi:10.3389/fphar.2022.1035969 | Review of preclinical data of PF-07304814 and its active metabolite derivatives against SARS-CoV-2 infection |
| Review of preclinical data for lufotrelvir and active metabolites for SARS-CoV-2. Lufotrelvir (PF-07304814) is a phosphate ester prodrug that is rapidly metabolized by alkaline phosphatase into the active compound PF-0835231, which suppre.. | ||
Oct 26 2022 |
et al., Clinical Pharmacology in Drug Development, doi:10.1002/cpdd.1174 | Safety, Tolerability, and Pharmacokinetics of Intravenous Doses of PF‐07304814, a Phosphate Prodrug Protease Inhibitor for the Treatment of SARS‐CoV‐2, in Healthy Adult Participants |
| Phase 1 randomized, double-blind, placebo-controlled study of 15 healthy participants showing safety and tolerability of single ascending 24-hour IV infusions of lufotrelvir (PF-07304814), a phosphate prodrug protease inhibitor targeting .. | ||