PF-07304814 for Inpatients With COVID-19 (An ACTIV-3/TICO Treatment Trial)
et al., NCT05780541, NCT05780541, Feb 2024
RCT 58 patients showing no significant differences with lufotrelvir. This is a 2021 study which has not been published yet. Results were made available on clinicaltrials.gov in 2024.
|
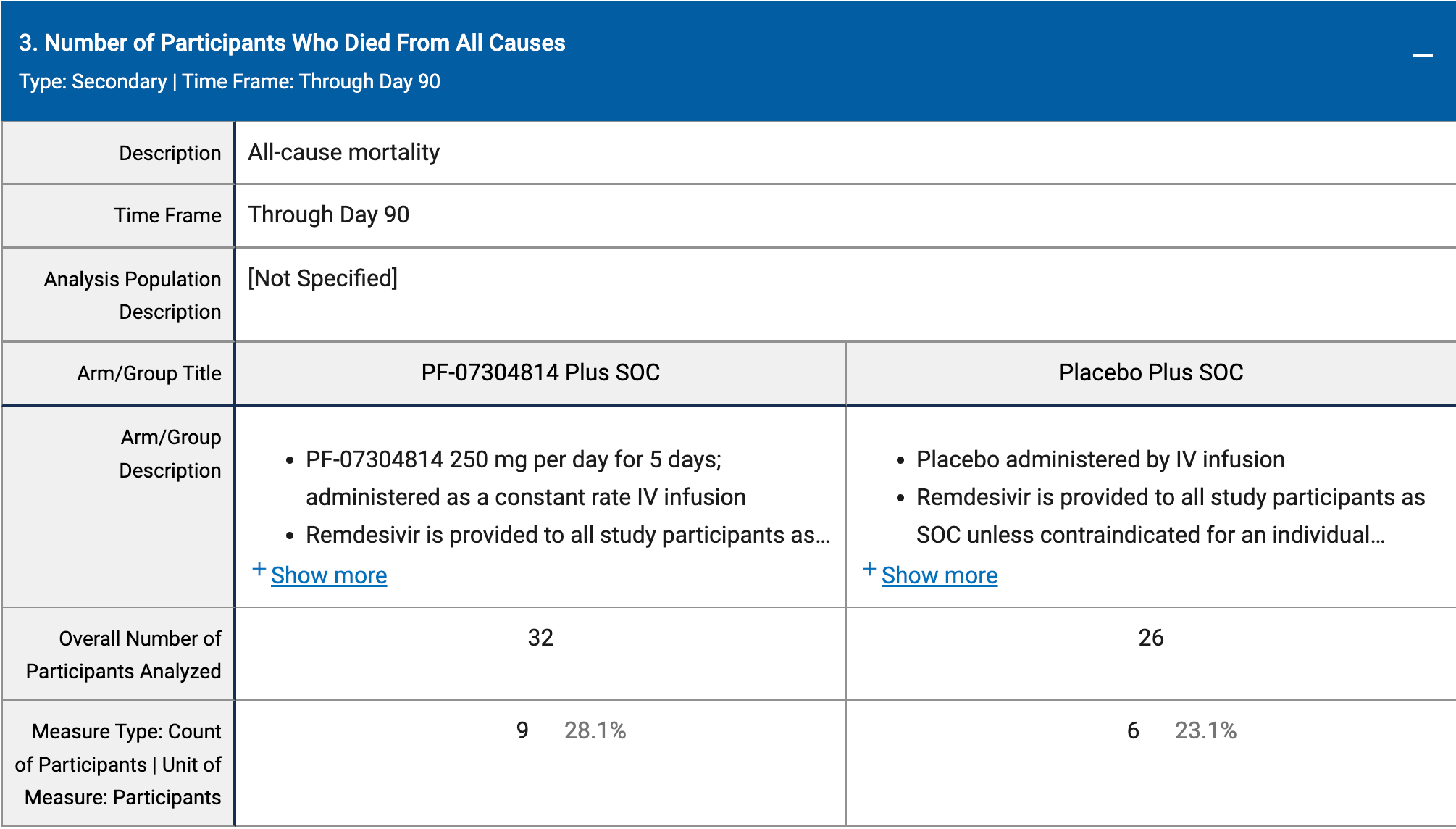
risk of death, 21.9% higher, RR 1.22, p = 0.77, treatment 9 of 32 (28.1%), control 6 of 26 (23.1%), day 90.
|
|
risk of no hospital discharge, 43.8% lower, RR 0.56, p = 0.11, treatment 9 of 32 (28.1%), control 13 of 26 (50.0%), NNT 4.6, day 90.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Grandits et al., 9 Feb 2024, Double Blind Randomized Controlled Trial, placebo-controlled, multiple countries, peer-reviewed, 1 author, trial NCT05780541 (history).