Bemnifosbuvir (AT-527) is an orally bioavailable double prodrug of a guanosine nucleotide analogue that, once converted intracellularly to AT-9010, inhibits SARS-CoV-2 replication through a dual mechanism: it plugs the catalytic site of the viral RNA-dependent RNA polymerase and occupies the NiRAN domain that is essential for RNA capping, thereby halting viral genome synthesis and maturation. In Vitro studies show sub-micromolar potency against multiple variants, and pharmacokinetic analysis shows that oral dosing achieves lung concentrations exceeding antiviral thresholds.
Feb 11 |
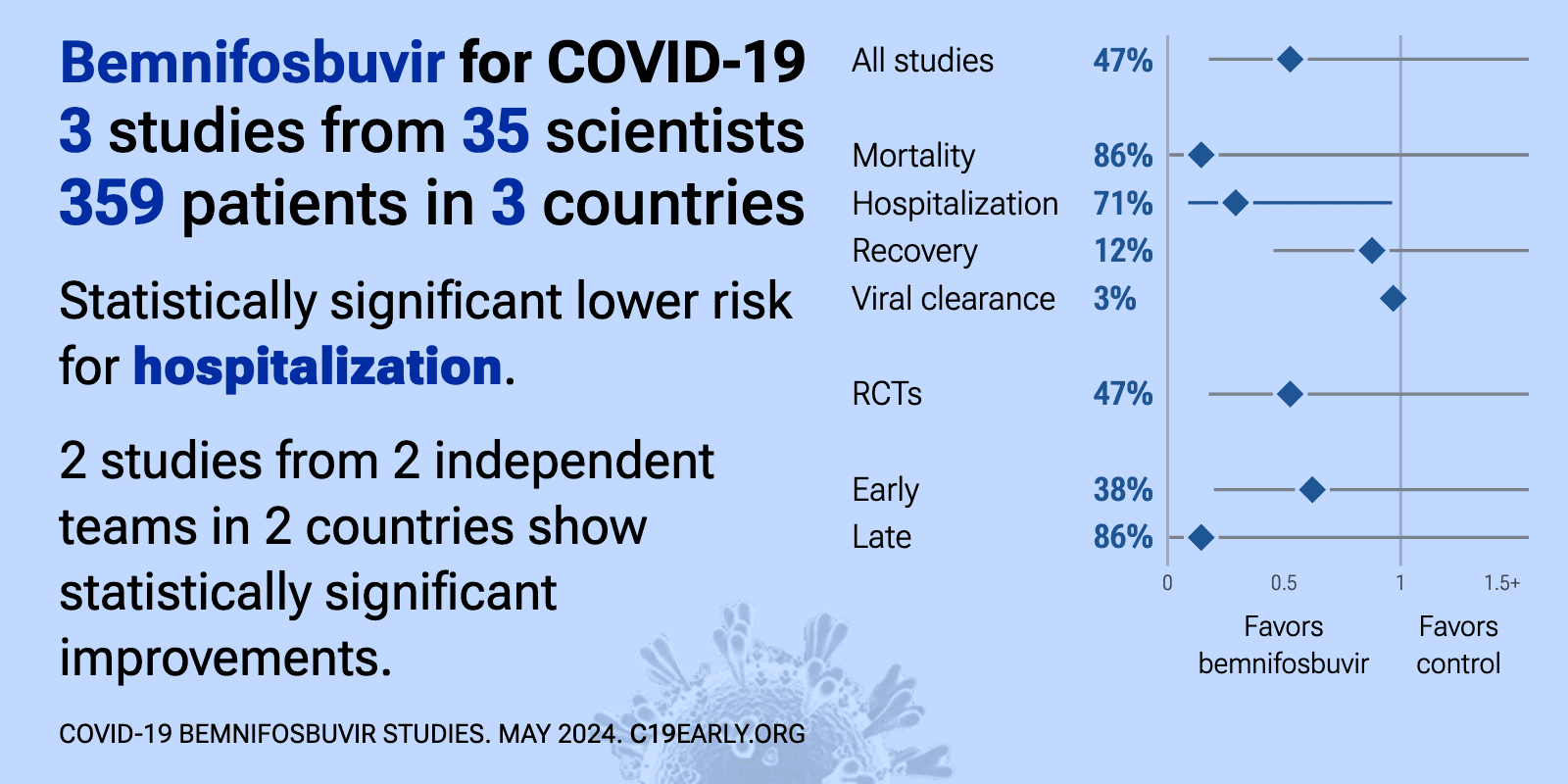
Bemnifosbuvir for COVID-19: real-time meta-analysis of 4 studies (Version 4) | |
| Meta-analysis using the most serious outcome reported shows 32% [-74‑73%] lower risk, without reaching statistical significance. Results are better for peer-reviewed studies. Currently all studies are RCTs. 2 studies (both from.. | ||
May 30 2024 |
et al., NCT05629962 | A Phase 3 Randomized, Double-Blind, Placebo-Controlled Study to Evaluate the Efficacy and Safety of Bemnifosbuvir in High-Risk Outpatients With COVID-19 |
| 202% higher mortality (p=0.37), 253% higher hospitalization (p=0.11), and 24% improvement (p=0.66). RCT 2,285 high-risk outpatients showing no significant difference in outcomes with bemnifosbuvir treatment. | ||
Jan 8 2024 |
et al., Journal of Antimicrobial Chemotherapy, doi:10.1093/jac/dkae122 (date from preprint) | Human bronchopulmonary disposition and plasma pharmacokinetics of oral bemnifosbuvir (AT-527), an experimental guanosine nucleotide prodrug for COVID-19 |
| Phase 1 study showing effective lung delivery and safety of the oral COVID-19 antiviral candidate bemnifosbuvir (AT-527) at 550mg twice daily. Authors found AT-527 550mg BID achieved sustained antiviral drug levels in lung fluids that exc.. | ||
Nov 1 2023 |
et al., Future Virology, doi:10.2217/fvl-2023-0115 | Oral bemnifosbuvir (AT-527) vs placebo in patients with mild-to-moderate COVID-19 in an outpatient setting (MORNINGSKY) |
| 71% lower hospitalization (p=0.05), 28% lower progression (p=0.49), 28% slower recovery, and 22% improved viral clearance (p=0.21). MORNINGSKY RCT which was terminated early after enrolling only 216 of 1,386 planned participants. The trial did not meet its primary endpoint, with the bemnifosbuvir group having longer time to symptom improvement than placebo. However, c.. | ||
Aug 17 2023 |
et al., Microbiology Spectrum, doi:10.1128/spectrum.00077-23 | A Phase 2 Randomized Trial Evaluating the Antiviral Activity and Safety of the Direct-Acting Antiviral Bemnifosbuvir in Ambulatory Patients with Mild or Moderate COVID-19 (MOONSONG Study) |
| 3% improved viral clearance (p=0.14). RCT 100 mild/moderate COVID-19 patients showing no significant difference in nasopharyngeal viral load reduction between bemnifosbuvir (550mg or 1100mg twice daily for 5 days) and placebo groups. | ||
Jun 23 2023 |
et al., Future Virology, doi:10.2217/fvl-2023-0064 | Phase II study of bemnifosbuvir in high-risk participants in a hospital setting with moderate COVID-19 |
| 86% lower mortality (p=0.24), 2% improved recovery (p=1), and 9% improved viral clearance (p=0.8). Phase 2 RCT investigating bemnifosbuvir for the treatment of 81 high-risk COVID-19 patients hospitalized with moderate disease. The trial was terminated early due to difficulties with enrollment. There was no significant difference betwee.. | ||