A Phase 3 Randomized, Double-Blind, Placebo-Controlled Study to Evaluate the Efficacy and Safety of Bemnifosbuvir in High-Risk Outpatients With COVID-19
et al., NCT05629962, SUNRISE-3, NCT05629962, May 2024
RCT 2,285 high-risk outpatients showing no significant difference in outcomes with bemnifosbuvir treatment.
Standard of Care (SOC) for COVID-19 in the study country,
the USA, is very poor with very low average efficacy for approved treatments1.
Only expensive, high-profit treatments were approved for early treatment. Low-cost treatments were excluded, reducing the probability of early treatment due to access and cost barriers, and eliminating complementary and synergistic benefits seen with many low-cost treatments.
|
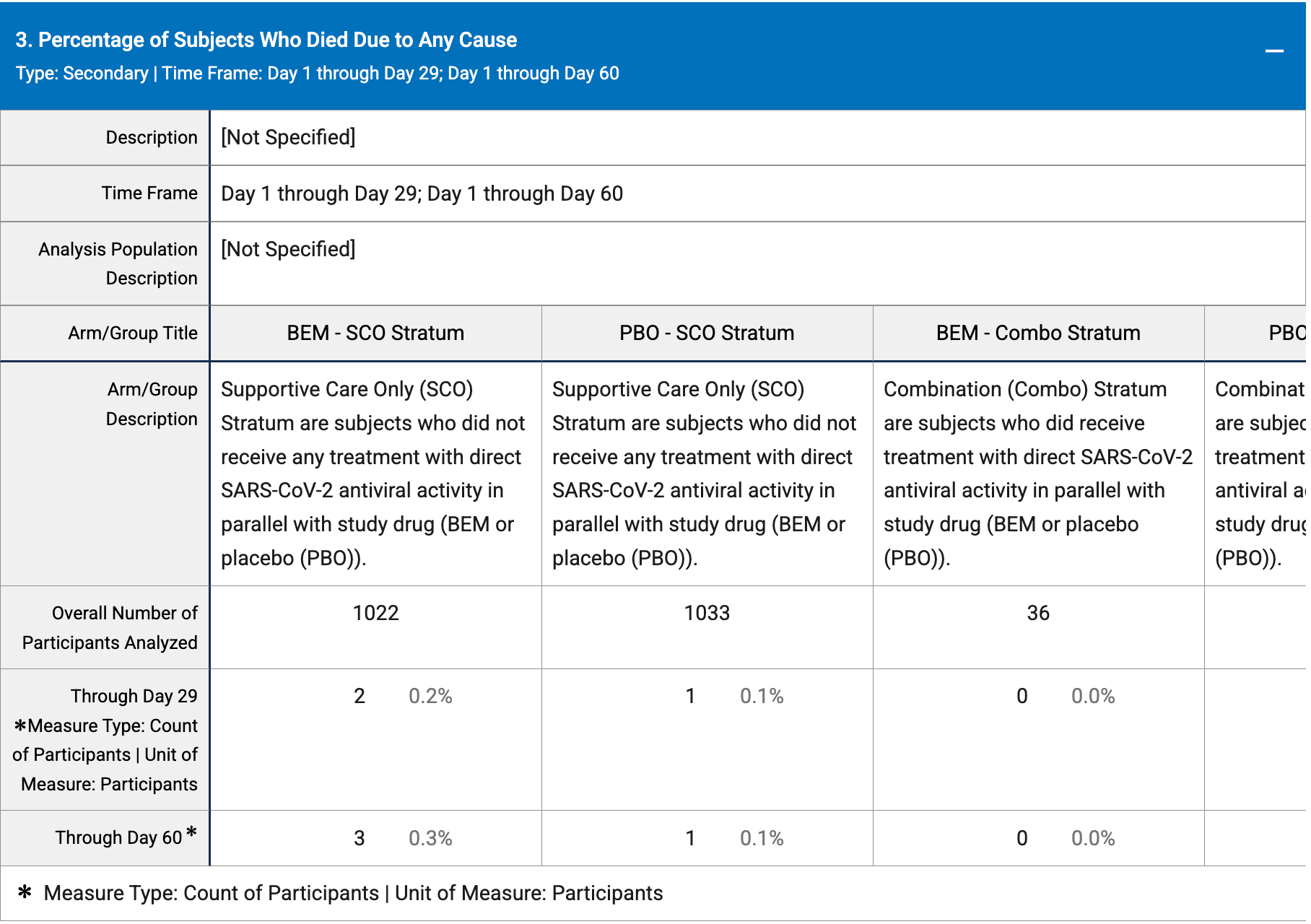
risk of death, 202.4% higher, RR 3.02, p = 0.37, treatment 3 of 1,130 (0.3%), control 1 of 1,139 (0.1%).
|
|
risk of hospitalization, 253.0% higher, RR 3.53, p = 0.11, treatment 7 of 1,058 (0.7%), control 2 of 1,067 (0.2%), all-cause hospitalization or mortality.
|
|
risk of hospitalization, 101.7% higher, RR 2.02, p = 0.45, treatment 4 of 1,058 (0.4%), control 2 of 1,067 (0.2%), COVID-19 hospitalization or all-cause mortality.
|
|
medical visits, 24.4% lower, RR 0.76, p = 0.66, treatment 9 of 1,058 (0.9%), control 12 of 1,067 (1.1%), NNT 365, medical visits or mortality.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Hammond et al., 30 May 2024, Double Blind Randomized Controlled Trial, placebo-controlled, USA, preprint, 1 author, trial NCT05629962 (history) (SUNRISE-3).
Contact: ateaclinicaltrials@ateapharma.com.