A Phase 2 Randomized Trial Evaluating the Antiviral Activity and Safety of the Direct-Acting Antiviral Bemnifosbuvir in Ambulatory Patients with Mild or Moderate COVID-19 (MOONSONG Study)
et al., Microbiology Spectrum, doi:10.1128/spectrum.00077-23, MOONSONG, NCT04709835, Aug 2023
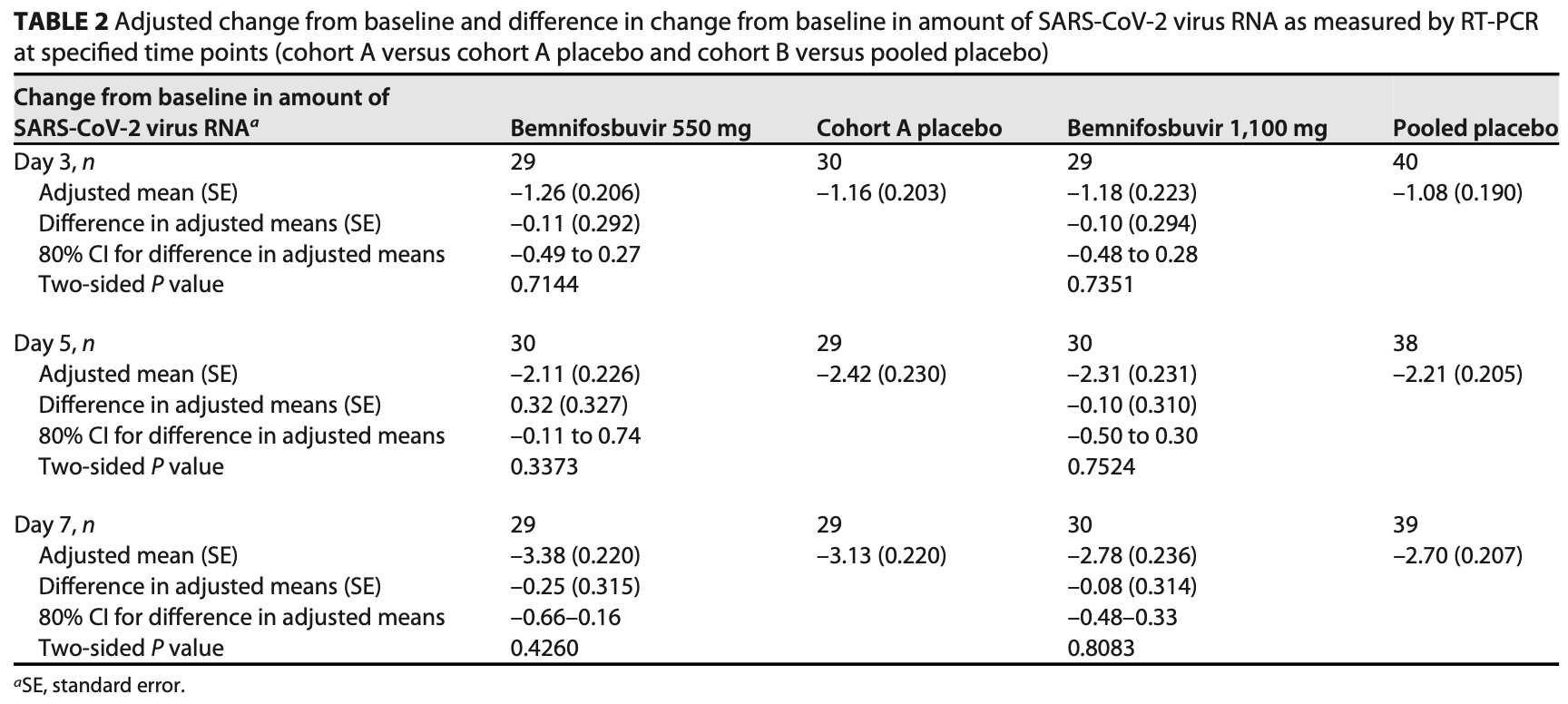
RCT 100 mild/moderate COVID-19 patients showing no significant difference in nasopharyngeal viral load reduction between bemnifosbuvir (550mg or 1100mg twice daily for 5 days) and placebo groups.
Meta-analysis of all bemnifosbuvir studies shows benefit for clinical outcomes but not for viral or case outcomes, consistent with an intervention that may have limited or no direct antiviral effect, but minimizes progression via other mechanisms (for example by aiding the immune system, minimizing immune over-activation, minizing secondary complications, or aiding recovery).
Standard of Care (SOC) for COVID-19 in the study country,
the United Kingdom, is very poor with very low average efficacy for approved treatments1.
The United Kingdom focused on expensive high-profit treatments, approving only one low-cost early treatment, which required a prescription and had limited adoption. The high-cost prescription treatment strategy reduces the probability of early treatment due to access and cost barriers, and eliminates complementary and synergistic benefits seen with many low-cost treatments.
|
viral load, 2.9% lower, relative load 0.97, p = 0.14, treatment mean 2.78 (±0.24) n=29, control mean 2.7 (±0.21) n=40, 1,100mg, day 7.
|
|
viral load, 7.4% lower, relative load 0.93, p < 0.001, treatment mean 3.38 (±0.22) n=29, control mean 3.13 (±0.22) n=29, 550mg, day 7.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Boffito et al., 17 Aug 2023, Double Blind Randomized Controlled Trial, placebo-controlled, United Kingdom, peer-reviewed, 11 authors, study period February 2021 - October 2021, trial NCT04709835 (history) (MOONSONG).
Contact: vincent.ukachukwu@roche.com, horga.arantxa@ateapharma.com.
A Phase 2 Randomized Trial Evaluating the Antiviral Activity and Safety of the Direct-Acting Antiviral Bemnifosbuvir in Ambulatory Patients with Mild or Moderate COVID-19 (MOONSONG Study)
Microbiology Spectrum, doi:10.1128/spectrum.00077-23
Bemnifosbuvir is an oral antiviral drug with a dual mechanism of action targeting viral RNA polymerase, with in vitro activity against SARS-CoV-2. We conducted a phase 2, double-blind study evaluating the antiviral activity, safety, efficacy, and pharmacokinetics of bemnifosbuvir in ambulatory patients with mild/moderate COVID-19. Patients were randomized 1:1 to bemnifosbuvir 550 mg or placebo (cohort A) and 3:1 to bemnifosbuvir 1,100 mg or placebo (cohort B); all doses were given twice daily for 5 days. The primary endpoint was a change from baseline in the amount of nasopharyngeal SARS-CoV-2 viral RNA by reverse transcription PCR (RT-PCR). The modified intent-to-treat infected population comprised 100 patients (bemnifosbuvir 550 mg, n = 30; bemnifosbuvir 1,100 mg, n = 30; cohort A placebo, n = 30; cohort B placebo, n = 10). The primary endpoint was not met: the difference in viral RNA adjusted means at day 7 was 20.25 log 10 copies/mL between bemnifosbuvir 550 mg and cohort A placebo (80% confidence interval [CI], 20.66 to 0.16; P = 0.4260), and 20.08 log 10 copies/mL between bemnifosbuvir 1,100 mg and pooled placebo (80% CI, 20.48 to 0.33; P = 0.8083). Bemnifosbuvir 550 mg was well tolerated. Incidence of nausea and vomiting was higher with bemnifosbuvir 1,100 mg (10.0% and 16.7% of patients, respectively) than pooled placebo (2.5% nausea, 2.5% vomiting). In the primary analysis, bemnifosbuvir did not show meaningful antiviral activity on nasopharyngeal viral load as measured by RT-PCR compared with placebo in patients with mild/moderate COVID-19. The trial is registered at ClinicalTrials.gov under registration number NCT04709835. IMPORTANCE COVID-19 continues to be a major global public health challenge, and there remains a need for effective and convenient direct-acting antivirals that can be administered outside health care settings. Bemnifosbuvir is an oral antiviral with a dual mechanism of action and potent in vitro activity against SARS-CoV-2. In this study, we evaluated the antiviral activity, safety, efficacy, and pharmacokinetics of bemnifosbuvir in ambulatory patients with mild/moderate COVID-19. In the primary analysis, bemnifosbuvir did not show meaningful antiviral activity compared with placebo as assessed by nasopharyngeal viral loads. The negative predictive value of nasopharyngeal viral load reduction for clinical outcomes in COVID-19 is currently unclear, and further evaluation of bemnifosbuvir for COVID-19 may be warranted despite the findings observed in this study.
SUPPLEMENTAL MATERIAL Supplemental material is available online only. SUPPLEMENTAL FILE 1, DOCX file, 2.2 MB.
References
Bergwerk, Gonen, Lustig, Amit, Lipsitch et al., Covid-19 breakthrough infections in vaccinated health care workers, N Engl J Med, doi:10.1056/NEJMoa2109072
Bernal, Da Silva, Musungaie, Kovalchuk, Gonzalez et al., Molnupiravir for oral treatment of Covid-19 in nonhospitalized patients, N Engl J Med, doi:10.1056/NEJMoa2116044
Fischer, Ii, Eron, Jr, Holman et al., A phase 2a clinical trial of molnupiravir in patients with COVID-19 shows accelerated SARS-CoV-2 RNA clearance and elimination of infectious virus, Sci Transl Med, doi:10.1126/scitranslmed.abl7430
Good, Westover, Jung, Zhou, Moussa et al., AT-527, a double prodrug of a guanosine nucleotide analog, is a potent inhibitor of SARS-CoV-2 in vitro and a promising oral antiviral for treatment of COVID-19, Antimicrob Agents Chemother, doi:10.1128/AAC.02479-20
Gottlieb, Vaca, Paredes, Mera, Webb et al., Early remdesivir to prevent progression to severe Covid-19 in outpatients, N Engl J Med, doi:10.1056/NEJMoa2116846
Gov, Uk, Government launches COVID-19 antivirals taskforce to roll out innovative home treatments this autumn
Hammond, Leister-Tebbe, Gardner, Abreu, Wisemandle et al., Oral nirmatrelvir for high-risk, nonhospitalized adults with Covid-19, N Engl J Med, doi:10.1056/NEJMoa2118542
Hayden, Sugaya, Hirotsu, Lee, De Jong et al., Baloxavir marboxil for uncomplicated influenza in adults and adolescents, N Engl J Med, doi:10.1056/NEJMoa1716197
Hijano, De Cardenas, Maron, Garner, Ferrolino et al., Clinical correlation of influenza and respiratory syncytial virus load measured by digital PCR, PLoS One, doi:10.1371/journal.pone.0220908
Hurt, Wheatley, Neutralizing antibody therapeutics for COVID-19, Viruses, doi:10.3390/v13040628
Lee, Wong, Chai, Lee, Lee et al., Efficacy of covid-19 vaccines in immunocompromised patients: systematic review and meta-analysis, BMJ, doi:10.1136/bmj-2021-068632
Lu, Wang, Sakthivel, Whitaker, Murray et al., US CDC real-time reverse transcription PCR panel for detection of severe acute respiratory syndrome coronavirus 2, Emerg Infect Dis, doi:10.3201/eid2608.201246
Mahase, Covid-19: where are we on vaccines and variants?, BMJ, doi:10.1136/bmj.n597
Paredes, Lunn, Famulare, Frisbie, Painter et al., Associations between severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) variants and risk of coronavirus disease 2019 (COVID-19) hospitalization among confirmed cases in Washington state: a retrospective cohort study, Clin Infect Dis, doi:10.1093/cid/ciac279
Razai, Chaudhry, Doerholt, Bauld, Majeed, Covid-19 vaccination hesitancy, BMJ, doi:10.1136/bmj.n1138
Salzberger, Buder, Lampl, Ehrenstein, Hitzenbichler et al., Epidemiology of SARS-CoV-2, Infection, doi:10.1007/s15010-020-01531-3
Shannon, Fattorini, Sama, Selisko, Feracci et al., Protein-primed RNA synthesis in SARS-CoVs and structural basis for inhibition by AT-527, BioRxiv, doi:10.1101/2021.03.23.436564
Somersan-Karakaya, Mylonakis, Menon, Wells, Ali et al., COVID-19 Phase 2/3 Hospitalized Trial Team. 2022. Casirivimab and imdevimab for the treatment of hospitalized patients with COVID-19, J Infect Dis jiac, doi:10.1093/infdis/jiac320
Tenforde, Self, Gaglani, Ginde, Douin et al., Effectiveness of mRNA vaccination in preventing COVID-19associated invasive mechanical ventilation and death: United States, March 2021-January 2022, MMWR Morb Mortal Wkly Rep, doi:10.15585/mmwr.mm7112e1
Wang, Nair, Liu, Iketani, Luo et al., Antibody resistance of SARS-CoV-2 variants B.1.351 and B.1.1.7, Nature, doi:10.1038/s41586-021-03398-2
Weinreich, Sivapalasingam, Norton, Ali, Gao et al., REGEN-COV antibody combination and outcomes in outpatients with Covid-19, N Engl J Med, doi:10.1056/NEJMoa2108163
Zhou, Horga, Puri, Montrond, Espié et al., AT-527 achieves antiviral concentrations in the human lung [poster 136
DOI record:
{
"DOI": "10.1128/spectrum.00077-23",
"ISSN": [
"2165-0497"
],
"URL": "http://dx.doi.org/10.1128/spectrum.00077-23",
"abstract": "<jats:p>\n COVID-19 continues to be a major global public health challenge, and there remains a need for effective and convenient direct-acting antivirals that can be administered outside health care settings. Bemnifosbuvir is an oral antiviral with a dual mechanism of action and potent\n <jats:italic>in vitro</jats:italic>\n activity against SARS-CoV-2.\n </jats:p>",
"alternative-id": [
"10.1128/spectrum.00077-23"
],
"assertion": [
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Received",
"name": "received",
"order": 0,
"value": "2023-01-17"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Accepted",
"name": "accepted",
"order": 1,
"value": "2023-05-26"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Published",
"name": "published",
"order": 2,
"value": "2023-06-20"
}
],
"author": [
{
"affiliation": [
{
"name": "Chelsea and Westminster Hospital NHS Foundation Trust London, United Kingdom"
},
{
"name": "Imperial College London, London, United Kingdom"
}
],
"family": "Boffito",
"given": "Marta",
"sequence": "first"
},
{
"affiliation": [
{
"name": "Connolly Hospital, Dublin, Ireland"
}
],
"family": "Dolan",
"given": "Eamon",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Tower Family Healthcare, Bury, United Kingdom"
}
],
"family": "Singh",
"given": "Karishma",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Roche Products Ltd., Welwyn Garden City, United Kingdom"
}
],
"family": "Holmes",
"given": "William",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Roche Pharma, Research and Early Development, Roche Innovation Center Basel, Basel, Switzerland"
}
],
"family": "Wildum",
"given": "Steffen",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Atea Pharmaceuticals, Boston, Massachusetts, USA"
}
],
"family": "Horga",
"given": "Arantxa",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Atea Pharmaceuticals, Boston, Massachusetts, USA"
}
],
"family": "Pietropaolo",
"given": "Keith",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Atea Pharmaceuticals, Boston, Massachusetts, USA"
}
],
"family": "Zhou",
"given": "Xiao-Jian",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Roche Products Ltd., Welwyn Garden City, United Kingdom"
}
],
"family": "Clinch",
"given": "Barry",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Roche Products Ltd., Welwyn Garden City, United Kingdom"
}
],
"family": "Collinson",
"given": "Neil",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-7663-9105",
"affiliation": [
{
"name": "Roche Products Ltd., Welwyn Garden City, United Kingdom"
}
],
"authenticated-orcid": true,
"family": "Ukachukwu",
"given": "Vincent",
"sequence": "additional"
}
],
"container-title": "Microbiology Spectrum",
"container-title-short": "Microbiol Spectr",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"journals.asm.org"
]
},
"created": {
"date-parts": [
[
2023,
6,
20
]
],
"date-time": "2023-06-20T14:05:53Z",
"timestamp": 1687269953000
},
"deposited": {
"date-parts": [
[
2023,
8,
17
]
],
"date-time": "2023-08-17T13:17:36Z",
"timestamp": 1692278256000
},
"editor": [
{
"affiliation": [],
"family": "Laeyendecker",
"given": "Oliver",
"sequence": "additional"
}
],
"funder": [
{
"DOI": "10.13039/100004337",
"doi-asserted-by": "publisher",
"name": "F. Hoffmann-La Roche Ltd"
},
{
"name": "Atea Pharmaceuticals"
}
],
"indexed": {
"date-parts": [
[
2023,
8,
18
]
],
"date-time": "2023-08-18T04:44:19Z",
"timestamp": 1692333859814
},
"is-referenced-by-count": 0,
"issue": "4",
"issued": {
"date-parts": [
[
2023,
8,
17
]
]
},
"journal-issue": {
"issue": "4",
"published-print": {
"date-parts": [
[
2023,
8,
17
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2023,
8,
17
]
],
"date-time": "2023-08-17T00:00:00Z",
"timestamp": 1692230400000
}
},
{
"URL": "https://journals.asm.org/non-commercial-tdm-license",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2023,
8,
17
]
],
"date-time": "2023-08-17T00:00:00Z",
"timestamp": 1692230400000
}
}
],
"link": [
{
"URL": "https://journals.asm.org/doi/pdf/10.1128/spectrum.00077-23",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://journals.asm.org/doi/pdf/10.1128/spectrum.00077-23",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "235",
"original-title": [],
"prefix": "10.1128",
"published": {
"date-parts": [
[
2023,
8,
17
]
]
},
"published-print": {
"date-parts": [
[
2023,
8,
17
]
]
},
"publisher": "American Society for Microbiology",
"reference": [
{
"key": "e_1_3_3_2_2",
"unstructured": "Johns Hopkins University. 2022. COVID-19 dashboard. https://coronavirus.jhu.edu/map.html. Accessed 3 October 2022."
},
{
"DOI": "10.1093/cid/ciac279",
"doi-asserted-by": "publisher",
"key": "e_1_3_3_3_2"
},
{
"DOI": "10.1007/s15010-020-01531-3",
"doi-asserted-by": "publisher",
"key": "e_1_3_3_4_2"
},
{
"key": "e_1_3_3_5_2",
"unstructured": "Centers for Disease Control and Prevention. 2022. Symptoms of COVID-19. https://www.cdc.gov/coronavirus/2019-ncov/symptoms-testing/symptoms.html. Accessed 3 October 2022."
},
{
"key": "e_1_3_3_6_2",
"unstructured": "European Medicines Agency. COVID-19 vaccines: key facts. https://www.ema.europa.eu/en/human-regulatory/overview/public-health-threats/coronavirus-disease-covid-19/treatments-vaccines/vaccines-covid-19/covid-19-vaccines-key-facts. Accessed 3 October 2021."
},
{
"key": "e_1_3_3_7_2",
"unstructured": "United States Food and Drug Administration. 2023. COVID-19 vaccines. https://www.fda.gov/emergency-preparedness-and-response/coronavirus-disease-2019-covid-19/covid-19-vaccines#authorized-vaccines. Accessed 12 June 2023."
},
{
"DOI": "10.3390/v13040628",
"doi-asserted-by": "publisher",
"key": "e_1_3_3_8_2"
},
{
"key": "e_1_3_3_9_2",
"unstructured": "European Medicines Agency. 2022. Paxlovid: summary of product characteristics. https://www.ema.europa.eu/en/medicines/human/EPAR/paxlovid. Accessed 3 October 2022."
},
{
"key": "e_1_3_3_10_2",
"unstructured": "European Medicines Agency. 2022. Veklury: summary of product characteristics. https://www.ema.europa.eu/en/medicines/human/EPAR/veklury. Accessed 3 October 2022."
},
{
"DOI": "10.15585/mmwr.mm7112e1",
"doi-asserted-by": "publisher",
"key": "e_1_3_3_11_2"
},
{
"DOI": "10.1056/NEJMoa2109072",
"doi-asserted-by": "publisher",
"key": "e_1_3_3_12_2"
},
{
"DOI": "10.1136/bmj-2021-068632",
"doi-asserted-by": "publisher",
"key": "e_1_3_3_13_2"
},
{
"key": "e_1_3_3_14_2",
"unstructured": "Centers for Disease Control and Prevention. 2022. Interim clinical considerations for use of COVID-19 vaccines in the United States. https://www.cdc.gov/vaccines/covid-19/clinical-considerations/interim-considerations-us.html#contraindications. Accessed 3 October 2022."
},
{
"DOI": "10.1136/bmj.n1138",
"doi-asserted-by": "publisher",
"key": "e_1_3_3_15_2"
},
{
"key": "e_1_3_3_16_2",
"unstructured": "GOV.UK. 2021. Government launches COVID-19 antivirals taskforce to roll out innovative home treatments this autumn. https://www.gov.uk/government/news/government-launches-covid-19-antivirals-taskforce-to-roll-out-innovative-home-treatments-this-autumn. Accessed 3 October 2022."
},
{
"DOI": "10.1136/bmj.n597",
"doi-asserted-by": "publisher",
"key": "e_1_3_3_17_2"
},
{
"DOI": "10.1038/s41586-021-03398-2",
"doi-asserted-by": "publisher",
"key": "e_1_3_3_18_2"
},
{
"key": "e_1_3_3_19_2",
"unstructured": "United States Food and Drug Administration. 2022. Fact sheet for healthcare providers: emergency use authorization for paxlovid. https://www.fda.gov/media/155050/download. Accessed 3 October 2022."
},
{
"key": "e_1_3_3_20_2",
"unstructured": "United States Food and Drug Administration. 2022. Veklury prescribing information. https://www.gilead.com/-/media/files/pdfs/medicines/covid-19/veklury/veklury_pi.pdf. Accessed 3 October 2022."
},
{
"key": "e_1_3_3_21_2",
"unstructured": "U.S. Food and Drug Administration. 2021. Coronavirus (COVID-19) update: FDA authorizes additional oral antiviral for treatment of COVID-19 in certain adults. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-additional-oral-antiviral-treatment-covid-19-certain. Accessed 3 October 2022."
},
{
"key": "e_1_3_3_22_2",
"unstructured": "Medicines and Healthcare Products Regulatory Agency. 2022. Lagevrio: summary of product characteristics. https://www.gov.uk/government/publications/regulatory-approval-of-lagevrio-molnupiravir/summary-of-product-characteristics-for-lagevrio. Accessed 3 October 2022."
},
{
"key": "e_1_3_3_23_2",
"unstructured": "World Health Organization. 2022. Therapeutics and COVID-19: living guideline 26 September 2022. https://www.who.int/publications/i/item/WHO-2019-nCoV-therapeutics-2022.5. Accessed 3 October 2022."
},
{
"DOI": "10.1056/NEJMoa2118542",
"doi-asserted-by": "publisher",
"key": "e_1_3_3_24_2"
},
{
"DOI": "10.1056/NEJMoa2108163",
"doi-asserted-by": "publisher",
"key": "e_1_3_3_25_2"
},
{
"DOI": "10.1056/NEJMoa1716197",
"doi-asserted-by": "publisher",
"key": "e_1_3_3_26_2"
},
{
"DOI": "10.1371/journal.pone.0220908",
"doi-asserted-by": "publisher",
"key": "e_1_3_3_27_2"
},
{
"DOI": "10.1056/NEJMoa2116044",
"doi-asserted-by": "publisher",
"key": "e_1_3_3_28_2"
},
{
"DOI": "10.1126/scitranslmed.abl7430",
"doi-asserted-by": "publisher",
"key": "e_1_3_3_29_2"
},
{
"DOI": "10.1093/infdis/jiac320",
"doi-asserted-by": "publisher",
"key": "e_1_3_3_30_2"
},
{
"DOI": "10.1056/NEJMoa2116846",
"doi-asserted-by": "publisher",
"key": "e_1_3_3_31_2"
},
{
"DOI": "10.1128/AAC.02479-20",
"doi-asserted-by": "publisher",
"key": "e_1_3_3_32_2"
},
{
"article-title": "AT-527 achieves antiviral concentrations in the human lung [poster 136]",
"author": "Zhou X-J",
"journal-title": "COVID-19, Influenza and RSV: Surveillance-Informed Prevention and Treatment (ISIRV-WHO Virtual Conference)",
"key": "e_1_3_3_33_2",
"unstructured": "Zhou X-J, Horga A, Puri A, Montrond M, Espié P, Pietropaolo K, Belanger B, Hammond J. 2021. AT-527 achieves antiviral concentrations in the human lung [poster 136]. COVID-19, Influenza and RSV: Surveillance-Informed Prevention and Treatment (ISIRV-WHO Virtual Conference), 19–21 October 2021.",
"year": "2021"
},
{
"DOI": "10.1101/2021.03.23.436564",
"doi-asserted-by": "crossref",
"key": "e_1_3_3_34_2",
"unstructured": "Shannon A Fattorini V Sama B Selisko B Feracci M Falcou C Gauffre P El Kazzi P Decroly E Rabah N Alvarez K Eydoux C Guillemot J-C Debart F Vasseur J-J Noel M Moussa A Good S Lin K Sommadossi J-P Zhu Y Yan X Shi H Ferron F Canard B. 2021. Protein-primed RNA synthesis in SARS-CoVs and structural basis for inhibition by AT-527. BioRxiv. doi:10.1101/2021.03.23.436564."
},
{
"DOI": "10.3201/eid2608.201246",
"doi-asserted-by": "publisher",
"key": "e_1_3_3_35_2"
},
{
"key": "e_1_3_3_36_2",
"unstructured": "U.S. Food and Drug Administration. 2020. Assessing COVID-19-related symptoms in outpatient adult and adolescent subjects in clinical trials of drugs and biological products for COVID-19 prevention or treatment: guidance for industry. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/assessing-covid-19-related-symptoms-outpatient-adult-and-adolescent-subjects-clinical-trials-drugs. Accessed 3 October 2022."
}
],
"reference-count": 35,
"references-count": 35,
"relation": {},
"resource": {
"primary": {
"URL": "https://journals.asm.org/doi/10.1128/spectrum.00077-23"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Infectious Diseases",
"Cell Biology",
"Microbiology (medical)",
"Genetics",
"General Immunology and Microbiology",
"Ecology",
"Physiology"
],
"subtitle": [],
"title": "A Phase 2 Randomized Trial Evaluating the Antiviral Activity and Safety of the Direct-Acting Antiviral Bemnifosbuvir in Ambulatory Patients with Mild or Moderate COVID-19 (MOONSONG Study)",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1128/asmj-crossmark-policy-page",
"volume": "11"
}