Oral bemnifosbuvir (AT-527) vs placebo in patients with mild-to-moderate COVID-19 in an outpatient setting (MORNINGSKY)
et al., Future Virology, doi:10.2217/fvl-2023-0115, MORNINGSKY, NCT04889040, Nov 2023
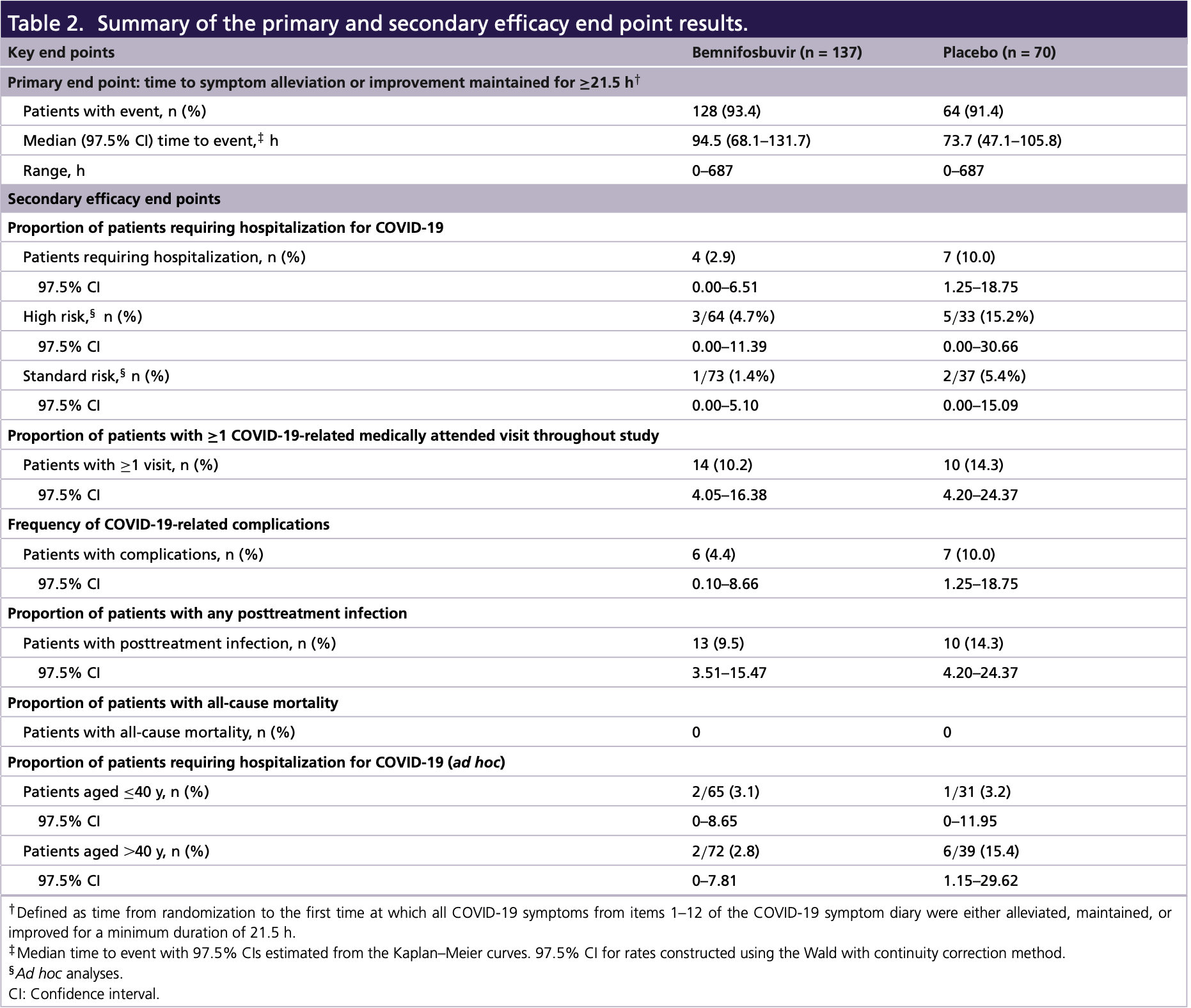
MORNINGSKY RCT which was terminated early after enrolling only 216 of 1,386 planned participants. The trial did not meet its primary endpoint, with the bemnifosbuvir group having longer time to symptom improvement than placebo. However, compared to placebo, bemnifosbuvir was associated with a 71% relative risk reduction in COVID-19 hospitalizations and fewer COVID-19 complications and medically attended visits, despite no significant improvement in viral load.
|
risk of hospitalization, 70.8% lower, RR 0.29, p = 0.047, treatment 4 of 137 (2.9%), control 7 of 70 (10.0%), NNT 14.
|
|
medical visit, 28.5% lower, RR 0.72, p = 0.49, treatment 14 of 137 (10.2%), control 10 of 70 (14.3%), NNT 25.
|
|
recovery time, 28.2% higher, relative time 1.28, treatment 137, control 70.
|
|
risk of no recovery, 23.4% lower, RR 0.77, p = 0.58, treatment 9 of 137 (6.6%), control 6 of 70 (8.6%), NNT 50.
|
|
risk of no viral clearance, 21.8% lower, RR 0.78, p = 0.21, treatment 44 of 128 (34.4%), control 29 of 66 (43.9%), NNT 10, day 14.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Horga et al., 1 Nov 2023, Double Blind Randomized Controlled Trial, placebo-controlled, Mexico, peer-reviewed, 16 authors, trial NCT04889040 (history) (MORNINGSKY).
Contact: horga.arantxa@ateapharma.com.
Oral bemnifosbuvir (AT-527) vs placebo in patients with mild-to-moderate COVID-19 in an outpatient setting (MORNINGSKY)
Future Virology, doi:10.2217/fvl-2023-0115
Aim: This phase III study assessed the efficacy/safety/antiviral activity/pharmacokinetics of bemnifosbuvir, a novel, oral nucleotide analog to treat COVID-19. Patients & methods: Outpatient adults/adolescents with mild-to-moderate COVID-19 were randomized 2:1 to bemnifosbuvir/placebo. Time to symptom alleviation/improvement (primary outcome), risk of hospitalization/death, viral load and safety were evaluated. Results: Although the study was discontinued prematurely and did not meet its primary end point, bemnifosbuvir treatment resulted in fewer hospitalizations (71% relative risk reduction), COVID-19related medically attended hospital visits, and COVID-19-related complications compared with placebo. No reduction in viral load was observed. The proportion of patients with adverse events was similar; no deaths occurred. Conclusion: Bemnifosbuvir showed hospitalization reduction in patients with variable disease progression risk and was well tolerated. Clinical Trial Registration: NCT04889040 (ClinicalTrials.gov). Tweetable abstract: #Bemnifosbuvir is a novel, oral, nonmutagenic, nonteratogenic nucleotide analog with low potential for drug-drug interactions or resistance. Bemnifosbuvir showed a 71% reduction in hospitalization for #COVID-19 despite no symptom alleviation/viral load reduction differences. @ateapharma
Bemnifosbuvir
Summary points • Hospitalization due to COVID-19 has placed a significant burden on healthcare systems. With the advent of new SARS-CoV-2 variants, vaccines and other treatment approaches have shown diminished efficacy. As the pandemic continues to evolve, safe, effective, direct-acting and convenient antiviral agents with broad utility are needed. • Bemnifosbuvir is an oral, antiviral guanosine analog that inhibits viral RNA polymerase via a dual mechanism of action. • This phase III study of bemnifosbuvir was terminated early, enrolling only 216 of the planned 1386 patients. It did not achieve its primary end point, with patients who received bemnifosbuvir experiencing longer median time to symptom alleviation/improvement than those who received placebo. • Compared with those in the placebo group, patients who received bemnifosbuvir experienced fewer hospitalizations, with risk reduction for all evaluable patients (71%) and for those aged >40 years (82%). • Bemnifosbuvir treatment also led to fewer COVID-19-related medically attended hospital visits and COVID-19-related complications. • Bemnifosbuvir was well tolerated; most adverse events were mild to moderate, and no deaths occurred. • No difference in viral load was observed between the bemnifosbuvir and placebo groups. • With its dual mechanism of action, in vitro activity against all reported SARS-CoV-2 strains, favorable safety profile, and low potential for drug-drug interactions,..
References
Andrews, Tessier, Stowe, Duration of protection against mild and severe disease by COVID-19 vaccines, N. Engl. J. Med
Batiha, Al-Kuraishy, Gareeb, Targeting of neuroinflammation by glibenclamide in COVID-19: old weapon from arsenal, Inflammopharmacology
Batiha, Al-Kuraishy, Gareeb, Youssef, El-Sherbeni et al., A perspective study of the possible impact of obeticholic acid against SARS-CoV-2 infection, Inflammopharmacology
Beigel, Tomashek, Dodd, Remdesivir for the treatment of COVID-19 -final report, N. Engl. J. Med
Bernal, Da Silva, Musungaie, Molnupiravir for oral treatment of COVID-19 in nonhospitalized patients, N. Engl. J. Med
Clinicaltrials, Gov, SUNRISE-3: efficacy and safety of bemnifosbuvir in high-risk outpatients with COVID-19
Clinicaltrials, Gov, Safety and efficacy of AT-527 in subjects with moderate coronavirus disease (COVID-19) in a hospital setting
Clinicaltrials, Gov, Study to evaluate the effects of AT-527 in non-hospitalized adult patients with mild or moderate COVID-19, NCT
Clinicaltrials, Gov, Study to evaluate the effects of RO7496998 (AT-527) in non-hospitalized adult and adolescent participants with mild or moderate COVID-19 (MORNINGSKY)
Desai, Gyawali, Endpoints used in phase III randomized controlled trials of treatment options for COVID-19, EClinicalMedicine
Fusco, Shea, Lin, Health outcomes and economic burden of hospitalized COVID-19 patients in the United States, J. Med. Econ
Ganatra, Dani, Ahmad, Oral nirmatrelvir and ritonavir in non-hospitalized vaccinated patients with Covid-19, Clin. Infect. Dis, doi:10.1093/cid/ciac673
Gottlieb, Vaca, Paredes, Early remdesivir to prevent progression to severe COVID-19 in outpatients, N. Engl. J. Med
Hammond, Leister-Tebbe, Gardner, Oral nirmatrelvir for high-risk, nonhospitalized adults with COVID-19, N. Engl. J. Med
Hogan, Duerr, Dimartino, Remdesivir resistance in transplant recipients with persistent COVID-19, Clin. Infect. Dis, doi:10.1093/cid/ciac769
Horga, Kuritzkes, Kowalczyk, Phase 2 study of bemnifosbuvir in high-risk participants in a hospital setting with moderate COVID-19
Huang, Bemnifosbuvir, BEM, AT-527), a potent inhibitor of SARS-CoV-2 variants of concern (VOC), and a promising oral antiviral with a high resistance barrier for treatment of COVID-19 and other coronavirus infections
Iuliano, Brunkard, Boehmer, Trends in disease severity and health care utilization during the early omicron variant period compared with previous SARS-CoV-2 high transmission periods -United States, December 2020-January 2022, MMWR Morb. Mortal. Wkly Rep
Jang, Choe, Yun, Reinfection with SARS-CoV-2 in general population, South Korea; nationwide retrospective cohort study, J. Med. Virol
Kuriakose, Singh, Pau, Developing treatment guidelines during a pandemic health crisis: lessons learned from COVID-19, Ann. Intern. Med
Lagevrio, Emergency Use Authorization Fact Sheet
Li, Hilgenfeld, Whitley, Clercq, Therapeutic strategies for COVID-19: progress and lessons learned, Nat. Rev. Drug Discov, doi:10.1038/s41573-023-00672-y1-27
Marquez, Kerkhoff, Schrom, COVID-19 symptoms and duration of direct antigen test positivity at a community testing and surveillance site, MedRxiv, doi:10.1101/2022.05.19.222749682022.2005.2019.22274968
Mathieu, Ritchie, Ortiz-Ospina, A global database of COVID-19 vaccinations, Nat. Hum. Behav
Molnupiravir, Nih, None
Montgomery, Hobbs, Padilla, Efficacy and safety of intramuscular administration of tixagevimab-cilgavimab for early outpatient treatment of COVID-19 (TACKLE): a phase 3, randomised, double-blind, placebo-controlled trial, Lancet Respir. Med, doi:10.1016/s2213-2600(22)00180-1
Pang, Xu, Liu, Li, Chen, The research progress of SARS-CoV-2 main protease inhibitors from 2020 to 2022, Eur. J. Med. Chem
Pepperrell, Ellis, Wang, Hill, Barriers to worldwide access for Paxlovid, a new treatment for COVID-19, Open Forum Infect. Dis
Salian, Wright, Vedell, COVID-19 transmission, current treatment, and future therapeutic strategies, Mol Pharm
Sanderson, Hisner, Ia, Peacock, Ruis, Identification of a molnupiravir-associated mutational signature in SARS-CoV-2 sequencing databases, medRxiv, doi:10.1101/2023.01.26.23284998
Shannon, Fattorini, Sama, A dual mechanism of action of AT-527 against SARS-CoV-2 polymerase, Nat. Commun
Steele, Couture, Reed, Estimated number of COVID-19 infections, hospitalizations, and deaths prevented among vaccinated persons in the US, December 2020 to September 2021, JAMA Netw. Open
Takashita, Kinoshita, Yamayoshi, Efficacy of antibodies and antiviral drugs against COVID-19 omicron variant, N. Engl. J. Med
Vallejos, Zoni, Bangher, Ivermectin to prevent hospitalizations in patients with COVID-19 (IVERCOR-COVID19) a randomized, double-blind, placebo-controlled trial, BMC Infect Dis
Veklury, Prescribing information
Vo, Good, Agrawal, Sommadossi, Low risk of drug-drug interactions (DDIs) for bemnifosbuvir (BEM) based upon in vitro metabolism and transporter interaction studies
Vuorio, Kovanen, Raal, Cholesterol-lowering drugs for high-risk hypercholesterolemia patients with COVID-19 while on Paxlovid™ therapy, Future Virol
Wang, Yang, Song, Oral GS-441524 derivatives: Next-generation inhibitors of SARS-CoV-2 RNA-dependent RNA polymerase, Front. Immunol
Wen, Chen, Tang, Efficacy and safety of three new oral antiviral treatment (molnupiravir, fluvoxamine and Paxlovid) for COVID-19: a meta-analysis, Ann. Med
Zhou, Horga, Puri, AT-527 achieves antiviral concentrations in the human lung
Zhou, Morelli, Montrond, Bemnifosbuvir has low potential to interfere with P-gp, BCRP, and OATP1B1-mediated transport
Zhou, Morelli, Montround, No dose adjustments for CYP3A4 substrates when co-administered with bemnifosbuvir
DOI record:
{
"DOI": "10.2217/fvl-2023-0115",
"ISSN": [
"1746-0794",
"1746-0808"
],
"URL": "http://dx.doi.org/10.2217/fvl-2023-0115",
"abstract": "<jats:p> Aim: This phase III study assessed the efficacy/safety/antiviral activity/pharmacokinetics of bemnifosbuvir, a novel, oral nucleotide analog to treat COVID-19. Patients & methods: Outpatient adults/adolescents with mild-to-moderate COVID-19 were randomized 2:1 to bemnifosbuvir/placebo. Time to symptom alleviation/improvement (primary outcome), risk of hospitalization/death, viral load and safety were evaluated. Results: Although the study was discontinued prematurely and did not meet its primary end point, bemnifosbuvir treatment resulted in fewer hospitalizations (71% relative risk reduction), COVID-19-related medically attended hospital visits, and COVID-19-related complications compared with placebo. No reduction in viral load was observed. The proportion of patients with adverse events was similar; no deaths occurred. Conclusion: Bemnifosbuvir showed hospitalization reduction in patients with variable disease progression risk and was well tolerated. </jats:p><jats:p> Clinical Trial Registration: NCT04889040 ( ClinicalTrials.gov ). </jats:p>",
"alternative-id": [
"10.2217/fvl-2023-0115"
],
"author": [
{
"affiliation": [
{
"name": "Atea Pharmaceuticals, Inc, Boston, MA 02110, USA"
}
],
"family": "Horga",
"given": "Arantxa",
"sequence": "first"
},
{
"affiliation": [
{
"name": "Genentech, Inc, San Francisco, CA 94080, USA"
}
],
"family": "Saenz",
"given": "Rebecca",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Karadeniz Technical University, Trabzon, 61080, Turkey"
}
],
"family": "Yilmaz",
"given": "Gürdal",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Köhler & Milstein Research, Anahuac-Mayab University, Mérida, 97308, Mexico"
}
],
"family": "Simón-Campos",
"given": "Abraham",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Atea Pharmaceuticals, Inc, Boston, MA 02110, USA"
}
],
"family": "Pietropaolo",
"given": "Keith",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "F. Hoffmann-La Roche Ltd, Basel, 4070, Switzerland"
}
],
"family": "Stubbings",
"given": "William J",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Roche Products Limited, Welwyn Garden City, AL7 1TW, Hertfordshire, UK"
}
],
"family": "Collinson",
"given": "Neil",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Atea Pharmaceuticals, Inc, Boston, MA 02110, USA"
}
],
"family": "Ishak",
"given": "Laura",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Roche Pharma Canada, Mississauga, ON, L5N 5M8, Ontario, Canada"
}
],
"family": "Zrinscak",
"given": "Barbara",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Atea Pharmaceuticals, Inc, Boston, MA 02110, USA"
}
],
"family": "Belanger",
"given": "Bruce",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Roche Products Limited, Welwyn Garden City, AL7 1TW, Hertfordshire, UK"
}
],
"family": "Granier",
"given": "Catherine",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Atea Pharmaceuticals, Inc, Boston, MA 02110, USA"
}
],
"family": "Lin",
"given": "Kai",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "F. Hoffmann-La Roche Ltd, Basel, 4070, Switzerland"
}
],
"family": "C Hurt",
"given": "Aeron",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Atea Pharmaceuticals, Inc, Boston, MA 02110, USA"
}
],
"family": "Zhou",
"given": "Xiao-Jian",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-9779-5740",
"affiliation": [
{
"name": "F. Hoffmann-La Roche Ltd, Basel, 4070, Switzerland"
}
],
"authenticated-orcid": false,
"family": "Wildum",
"given": "Steffen",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Atea Pharmaceuticals, Inc, Boston, MA 02110, USA"
}
],
"family": "Hammond",
"given": "Janet",
"sequence": "additional"
}
],
"container-title": "Future Virology",
"container-title-short": "Future Virology",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2023,
11,
1
]
],
"date-time": "2023-11-01T06:01:39Z",
"timestamp": 1698818499000
},
"deposited": {
"date-parts": [
[
2023,
11,
1
]
],
"date-time": "2023-11-01T06:01:57Z",
"timestamp": 1698818517000
},
"funder": [
{
"name": "Atea Pharmaceuticals, Inc."
}
],
"indexed": {
"date-parts": [
[
2023,
11,
2
]
],
"date-time": "2023-11-02T04:17:16Z",
"timestamp": 1698898636345
},
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2023,
11,
1
]
]
},
"language": "en",
"link": [
{
"URL": "https://www.futuremedicine.com/doi/pdf/10.2217/fvl-2023-0115",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "1057",
"original-title": [],
"prefix": "10.2217",
"published": {
"date-parts": [
[
2023,
11,
1
]
]
},
"published-online": {
"date-parts": [
[
2023,
11,
1
]
]
},
"publisher": "Future Medicine Ltd",
"reference": [
{
"DOI": "10.1021/acs.molpharmaceut.0c00608",
"doi-asserted-by": "publisher",
"key": "B1"
},
{
"DOI": "10.1080/13696998.2021.1886109",
"doi-asserted-by": "publisher",
"key": "B2"
},
{
"DOI": "10.15585/mmwr.mm7104e4",
"doi-asserted-by": "publisher",
"key": "B3"
},
{
"DOI": "10.1038/s41562-021-01122-8",
"doi-asserted-by": "publisher",
"key": "B4"
},
{
"DOI": "10.1001/jamanetworkopen.2022.20385",
"doi-asserted-by": "publisher",
"key": "B5"
},
{
"DOI": "10.1002/jmv.28026",
"doi-asserted-by": "publisher",
"key": "B6"
},
{
"DOI": "10.1056/NEJMoa2115481",
"doi-asserted-by": "publisher",
"key": "B7"
},
{
"DOI": "10.1016/j.ejmech.2023.115491",
"doi-asserted-by": "publisher",
"key": "B8"
},
{
"key": "B9",
"unstructured": "World Health Organization. Therapeutics and COVID-19: living guideline by World Health Organization (WHO) (2021). https://apps.who.int/iris/handle/10665/345356"
},
{
"DOI": "10.7326/M21-1647",
"doi-asserted-by": "publisher",
"key": "B10"
},
{
"author": "Li G",
"first-page": "1",
"journal-title": "Nat. Rev. Drug Discov.",
"key": "B11",
"year": "2023"
},
{
"key": "B12",
"unstructured": "World Health Organization. Therapeutics and COVID-19: Living Guideline (2022). https://apps.who.int/iris/handle/10665/353403"
},
{
"key": "B13",
"unstructured": "VEKLURY. Prescribing information. Gilead Sciences, Inc (2022)."
},
{
"DOI": "10.3389/fimmu.2022.1015355",
"doi-asserted-by": "publisher",
"key": "B14"
},
{
"author": "Hogan JI",
"journal-title": "Clin. Infect. Dis.",
"key": "B15",
"year": "2022"
},
{
"key": "B16",
"unstructured": "LAGEVRIO. Emergency Use Authorization Fact Sheet. Merck Sharp & Dohme LLC (2022)."
},
{
"key": "B17",
"unstructured": "PAXLOVID. Emergency Use Authorization Fact Sheet. Pfizer Inc (2022)."
},
{
"DOI": "10.1080/07853890.2022.2034936",
"doi-asserted-by": "publisher",
"key": "B18"
},
{
"DOI": "10.1093/ofid/ofac174",
"doi-asserted-by": "publisher",
"key": "B19"
},
{
"key": "B20",
"unstructured": "Molnupiravir. NIH (2023). www.covid19treatmentguidelines.nih.gov/therapies/antivirals-including-antibody-products/molnupiravir/"
},
{
"author": "Sanderson T",
"journal-title": "medRxiv",
"key": "B21",
"year": "2023"
},
{
"DOI": "10.2217/fvl-2022-0060",
"doi-asserted-by": "publisher",
"key": "B22"
},
{
"DOI": "10.1007/s10787-022-01087-8",
"doi-asserted-by": "publisher",
"key": "B23"
},
{
"DOI": "10.1007/s10787-022-01111-x",
"doi-asserted-by": "publisher",
"key": "B24"
},
{
"key": "B25",
"unstructured": "Atea to advance global phase 3 registrational study of bemnifosbuvir in high-risk non-hospitalized patients with COVID-19. Press Release. Atea Pharmaceuticals (2022). https://ir.ateapharma.com/news-releases/news-release-details/atea-advance-global-phase-3-registrational-study-bemnifosbuvir"
},
{
"DOI": "10.1038/s41467-022-28113-1",
"doi-asserted-by": "publisher",
"key": "B26"
},
{
"DOI": "10.1056/NEJMc2119407",
"doi-asserted-by": "publisher",
"key": "B27"
},
{
"key": "B28",
"unstructured": "Atea Pharmaceuticals reports third quarter 2022 financial results and provides business update. Press Release. Atea Pharmaceuticals (2022). https://ir.ateapharma.com/news-releases/news-release-details/atea-pharmaceuticals-reports-third-quarter-2022-financial"
},
{
"key": "B29",
"unstructured": "Atea Pharmaceuticals reports first quarter 2023 financial results and provides business update. News release. Atea Pharmaceuticals (2023). https://ir.ateapharma.com/news-releases/news-release-details/atea-pharmaceuticals-reports-first-quarter-2023-financial"
},
{
"author": "Huang Q",
"key": "B30",
"volume-title": "Lecture presented at: International Conference on Antiviral Research.",
"year": "2023"
},
{
"author": "Zhou XJ",
"key": "B31",
"volume-title": "Poster presented at: the 2021 ISIRV-WHO Conference",
"year": "2021"
},
{
"key": "B32",
"unstructured": "Atea Pharmaceuticals provides update and topline results for phase 2 MOONSONG trial evaluating AT-527 in the outpatient setting. Press Release. Atea Pharmaceuticals (2021). https://ir.ateapharma.com/news-releases/news-release-details/atea-pharmaceuticals-provides-update-and-topline-results-phase-2"
},
{
"key": "B33",
"unstructured": "Atea Pharmaceuticals reports first quarter 2022 financial results and provides business update. Press Release. Atea Pharmaceuticals (2022). https://ir.ateapharma.com/news-releases/news-release-details/atea-pharmaceuticals-reports-first-quarter-2022-financial"
},
{
"key": "B34",
"unstructured": "Clinicaltrials.Gov. Safety and efficacy of AT-527 in subjects with moderate coronavirus disease (COVID-19) in a hospital setting. NCT04396106 (2020). https://clinicaltrials.gov/ct2/show/NCT04396106"
},
{
"key": "B35",
"unstructured": "Clinicaltrials.Gov. Study to evaluate the effects of AT-527 in non-hospitalized adult patients with mild or moderate COVID-19. NCT04709835 (2021). https://clinicaltrials.gov/ct2/show/NCT04709835"
},
{
"DOI": "10.2217/fvl-2023-0064",
"doi-asserted-by": "publisher",
"key": "B36"
},
{
"author": "Vo A",
"key": "B37",
"volume-title": "Poster presented at: International Conference on Antiviral Research.",
"year": "2023"
},
{
"author": "Zhou X-J",
"key": "B38",
"volume-title": "Poster presented at: Conference on Retroviruses and Opportunistic Infections.",
"year": "2023"
},
{
"author": "Zhou XJ",
"key": "B39",
"volume-title": "Poster presented at: Conference on Retroviruses and Opportunistic Infections.",
"year": "2023"
},
{
"key": "B40",
"unstructured": "ClinicalTrials.gov. Study to evaluate the effects of RO7496998 (AT-527) in non-hospitalized adult and adolescent participants with mild or moderate COVID-19 (MORNINGSKY). NCT04889040 (2021). https://clinicaltrials.gov/ct2/show/NCT04889040"
},
{
"key": "B41",
"unstructured": "US Food and Drug Administration. COVID-19: developing drugs and biological products for treatment or prevention: guidance for industry (2021). www.fda.gov/drugs/guidance-compliance-regulatory-information/guidances-drugs"
},
{
"key": "B42",
"unstructured": "US Food and Drug Administration. Assessing COVID-19-related symptoms in outpatient adult and adolescent subjects in clinical trials of drugs and biological products for COVID-19 prevention or treatment. Guidance for Industry (2020). www.fda.gov/regulatory-information/search-fda-guidance-documents/assessing-covid-19-related-symptoms-outpatient-adult-and-adolescent-subjects-clinical-trials-drugs"
},
{
"DOI": "10.1056/NEJMoa2116044",
"doi-asserted-by": "publisher",
"key": "B43"
},
{
"DOI": "10.1056/NEJMoa2118542",
"doi-asserted-by": "publisher",
"key": "B44"
},
{
"DOI": "10.1186/s12879-021-06348-5",
"doi-asserted-by": "publisher",
"key": "B45"
},
{
"author": "Marquez C",
"journal-title": "MedRxiv",
"key": "B46",
"year": "2022"
},
{
"DOI": "10.1056/NEJMoa2116846",
"doi-asserted-by": "publisher",
"key": "B47"
},
{
"author": "Ganatra S",
"journal-title": "Clin. Infect. Dis.",
"key": "B48",
"year": "2022"
},
{
"key": "B49",
"unstructured": "Pfizer reports additional data on Paxlovid supporting upcoming new drug application submission to U.S. FDA. Business Wire (2022). www.businesswire.com/news/home/20220613005755/en/"
},
{
"key": "B50",
"unstructured": "Centers for Disease Control and Prevention. COVID-19 rebound after Paxlovid treatment (2022). https://emergency.cdc.gov/han/2022/pdf/CDC_HAN_467.pdf"
},
{
"key": "B51",
"unstructured": "Atea's AT-527, an oral antiviral drug candidate, reduces viral replication in hospitalized patients with COVID-19 in phase 2 interim analysis. Atea Pharmaceuticals (2021). https://ir.ateapharma.com/news-releases/news-release-details/ateas-527-oral-antiviral-drug-candidate-reduces-viral"
},
{
"key": "B52",
"unstructured": "AstraZeneca. Evusheld significantly prevented COVID-19 disease progression or death in TACKLE phase III treatment trial. Press release (2022). www.astrazeneca.com/media-centre/press-releases/2022/evusheld-significantly-prevented-covid-19-disease-progression-or-death-in-tackle-phase-iii-treatment-trial.html"
},
{
"key": "B53",
"unstructured": "Adagio Therapeutics announces ADG20 (adintrevimab) is the first monoclonal antibody to meet primary endpoints with statistical significance across pre- and post-exposure prophylaxis and treatment for COVID-19 and plans to seek U.S. emergency use authorization. Globe Newswire (2022). https://investors.adagiotx.com/news-releases/news-release-details/adagio-therapeutics-announces-adg20-adintrevimab-first"
},
{
"DOI": "10.1016/S2213-2600(22)00180-1",
"doi-asserted-by": "publisher",
"key": "B54"
},
{
"DOI": "10.1016/j.eclinm.2020.100403",
"doi-asserted-by": "publisher",
"key": "B55"
},
{
"DOI": "10.1056/NEJMoa2007764",
"doi-asserted-by": "publisher",
"key": "B56"
},
{
"key": "B57",
"unstructured": "Atea Pharmaceuticals announces U.S. FDA fast track designation granted to bemnifosbuvir, an investigational oral antiviral, for the treatment of COVID-19. News release. Atea pharmaceuticals (2023). https://ir.ateapharma.com/news-releases/news-release-details/atea-pharmaceuticals-announces-us-fda-fast-track-designation-0"
},
{
"key": "B58",
"unstructured": "Clinicaltrials.Gov. SUNRISE-3: efficacy and safety of bemnifosbuvir in high-risk outpatients with COVID-19. NCT05629962 (2022). https://clinicaltrials.gov/ct2/show/NCT05629962"
}
],
"reference-count": 58,
"references-count": 58,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.futuremedicine.com/doi/10.2217/fvl-2023-0115"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Virology"
],
"subtitle": [],
"title": "Oral bemnifosbuvir (AT-527) vs placebo in patients with mild-to-moderate COVID-19 in an outpatient setting (MORNINGSKY)",
"type": "journal-article"
}