Oral Nirmatrelvir and Ritonavir in Non-hospitalized Vaccinated Patients with Covid-19
et al., Clinical Infectious Diseases, doi:10.1093/cid/ciac673, Aug 2022
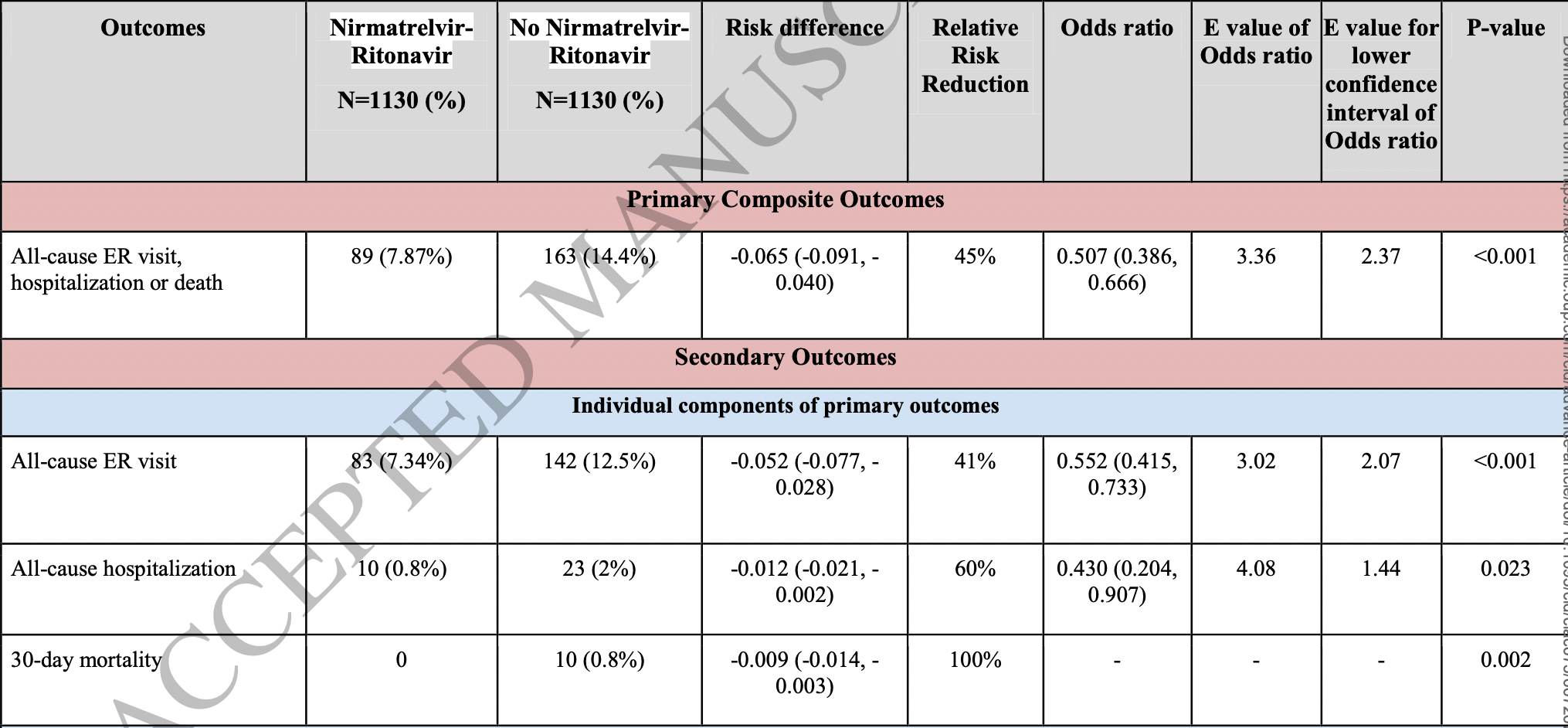
TriNetX retrospective 1,131 vaccinated COVID-19 patients treated with paxlovid and matched controls, showing lower mortality and hospitalization with treatment.
Confounding by treatment propensity. This study analyzes a population
where only a fraction of eligible patients received the treatment. Patients
receiving treatment may be more likely to follow other recommendations, more
likely to receive additional care, and more likely to use additional
treatments that are not tracked in the data (e.g., nasal/oral hygiene1,2, vitamin D3, etc.) — either because the physician
recommending paxlovid also recommended them, or
because the patient seeking out paxlovid is more
likely to be familiar with the efficacy of additional treatments and more
likely to take the time to use them.
Malden et al. confirm significant bias in the use of paxlovid, showing that treated
patients are more likely to be from affluent neighborhoods, be more health-conscious, and
have better access to care. Campion et al. also show that female patients were more
likely to receive paxlovid, and studies show that female patients are significantly more
likely to be health-conscious, for example being more likely to take additional
non-prescription treatments.
Therefore, these kind of studies may
overestimate efficacy.
Resistance. Variants may be resistant to paxlovid6-13. Use may promote the emergence of variants that weaken host immunity and potentially contribute to long COVID14. Confounding by contraindication. Hoertel et al. find that over 50% of patients that died had a contraindication for the use of Paxlovid15. Retrospective studies that do not exclude contraindicated patients may significantly overestimate efficacy. Black box warning. The FDA notes that severe, life-threatening, and/or fatal adverse reactions due to drug interactions have been reported in patients treated with paxlovid16. Kidney and liver injury. Studies show significantly increased risk of acute kidney injury17 and liver injury18,19. Viral rebound. Studies show significantly increased risk of replication-competent viral rebound20-22.
Standard of Care (SOC) for COVID-19 in the study country,
the USA, is very poor with very low average efficacy for approved treatments23.
Only expensive, high-profit treatments were approved for early treatment. Low-cost treatments were excluded, reducing the probability of early treatment due to access and cost barriers, and eliminating complementary and synergistic benefits seen with many low-cost treatments.
This study is excluded in the after exclusion results of meta-analysis:
only a fraction of eligible patients received treatment and these patients may be more likely to follow other recommendations, receive additional care, and more more likely to use additional untracked treatments such as vitamin D and nasal/oral hygiene.
|
risk of death, 95.2% lower, RR 0.05, p = 0.002, treatment 0 of 1,130 (0.0%), control 10 of 1,130 (0.9%), NNT 113, relative risk is not 0 because of continuity correction due to zero events (with reciprocal of the contrasting arm), propensity score matching, day 30.
|
|
risk of progression, 39.2% lower, RR 0.61, p < 0.001, treatment 89 of 1,130 (7.9%), control 163 of 1,130 (14.4%), NNT 15, odds ratio converted to relative risk, combined ER/hospitalization/death, propensity score matching, day 30.
|
|
risk of progression, 32.9% lower, HR 0.67, p = 0.003, treatment 89 of 1,130 (7.9%), control 163 of 1,130 (14.4%), combined ER/hospitalization/death, propensity score matching, Kaplan-Meier, day 30.
|
|
risk of hospitalization, 56.5% lower, RR 0.44, p = 0.02, treatment 10 of 1,130 (0.9%), control 23 of 1,130 (2.0%), NNT 87, odds ratio converted to relative risk, propensity score matching, day 30.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
4.
Malden et al., Predictors of nirmatrelvir–ritonavir receipt among COVID-19 patients in a large US health system, Scientific Reports, doi:10.1038/s41598-024-57633-7.
5.
Campion et al., Disparities in the Use of nirmatrelvir/ritonavir for COVID-19: A Retrospective Cohort Study, Open Forum Infectious Diseases, doi:10.1093/ofid/ofaf695.1809.
6.
Zhou et al., Nirmatrelvir-resistant SARS-CoV-2 variants with high fitness in an infectious cell culture system, Science Advances, doi:10.1126/sciadv.add7197.
7.
Moghadasi et al., Rapid resistance profiling of SARS-CoV-2 protease inhibitors, npj Antimicrobials and Resistance, doi:10.1038/s44259-023-00009-0.
8.
Jochmans et al., The Substitutions L50F, E166A, and L167F in SARS-CoV-2 3CLpro Are Selected by a Protease Inhibitor In Vitro and Confer Resistance To Nirmatrelvir, mBio, doi:10.1128/mbio.02815-22.
9.
Lopez et al., SARS-CoV-2 Resistance to Small Molecule Inhibitors, Current Clinical Microbiology Reports, doi:10.1007/s40588-024-00229-6.
10.
Zvornicanin et al., Molecular Mechanisms of Drug Resistance and Compensation in SARS-CoV-2 Main Protease: The Interplay Between E166 and L50, bioRxiv, doi:10.1101/2025.01.24.634813.
11.
Vukovikj et al., Impact of SARS-CoV-2 variant mutations on susceptibility to monoclonal antibodies and antiviral drugs: a non-systematic review, April 2022 to October 2024, Eurosurveillance, doi:10.2807/1560-7917.ES.2025.30.10.2400252.
12.
Deschenes et al., Functional and structural characterization of treatment-emergent nirmatrelvir resistance mutations at low frequencies in the main protease (Mpro) reveals a unique evolutionary route for SARS-CoV-2 to gain resistance, The Journal of Infectious Diseases, doi:10.1093/infdis/jiaf294.
13.
Zhou (B) et al., SARS-CoV-2 Mpro inhibitor ensitrelvir: asymmetrical cross-resistance with nirmatrelvir and emerging resistance hotspots, Emerging Microbes & Infections, doi:10.1080/22221751.2025.2552716.
14.
Thomas et al., Nirmatrelvir-Resistant Mutations in SARS-CoV-2 Mpro Enhance Host Immune Evasion via Cleavage of NF-κB Essential Modulator, bioRxiv, doi:10.1101/2024.10.18.619137.
15.
Hoertel et al., Prevalence of Contraindications to Nirmatrelvir-Ritonavir Among Hospitalized Patients With COVID-19 at Risk for Progression to Severe Disease, JAMA Network Open, doi:10.1001/jamanetworkopen.2022.42140.
16.
FDA, Fact sheet for healthcare providers: emergency use authorization for paxlovid, www.fda.gov/media/155050/download.
17.
Kamo et al., Association of Antiviral Drugs for the Treatment of COVID-19 With Acute Renal Failure, In Vivo, doi:10.21873/invivo.13637.
18.
Wang et al., Development and validation of a nomogram to assess the occurrence of liver dysfunction in patients with COVID-19 pneumonia in the ICU, BMC Infectious Diseases, doi:10.1186/s12879-025-10684-1.
19.
Siby et al., Temporal Trends in Serious Adverse Events Associated with Oral Antivirals During the COVID-19 Pandemic: Insights from the FAERS Database (2020–2023), Open Forum Infectious Diseases, doi:10.1093/ofid/ofaf695.1825.
20.
Edelstein et al., SARS-CoV-2 virologic rebound with nirmatrelvir-ritonavir therapy, medRxiv, doi:10.1101/2023.06.23.23288598.
21.
Shah et al., SARS-CoV-2 infectious shedding and rebound among adults with and without oral antiviral use: two case-ascertained prospective household studies, The Lancet Microbe, doi:10.1016/j.lanmic.2025.101227.
Ganatra et al., 20 Aug 2022, retrospective, USA, peer-reviewed, mean age 57.6, 9 authors, study period 1 December, 2021 - 18 April, 2022.
Contact: sarju.ganatra@lahey.org.
Oral Nirmatrelvir and Ritonavir in Non-hospitalized Vaccinated Patients with Covid-19
doi:10.1093/cid/ciac673/6672670
Background Treatment of coronavirus disease-2019 (Covid-19) with nirmatrelvir plus ritonavir (NMV-r) in high-risk non-hospitalized unvaccinated patients reduced the risk of progression to severe disease. However, the potential benefits of NMV-r among vaccinated patients are unclear.
Methods We conducted a comparative retrospective cohort study using the TriNetX research network. Patients ≥18 years of age who were vaccinated and subsequently developed Covid-19 between December 1, 2021, and April 18, 2022, were included. Cohorts were developed based on the use of NMV-r within five days of diagnosis. The primary composite outcome was all-cause emergency room (ER) visit, hospitalization, or death at a 30-days follow-up. Secondary outcomes included individual components of primary outcomes, multisystem symptoms, Covid-19 associated complications, and diagnostic test utilization.
Results After propensity score matching, 1,130 patients remained in each cohort. A primary composite outcome of all-cause ER visits, hospitalization, or death in 30 days occurred in 89 (7.87%) patients in the NMV-r cohort as compared to 163 (14.4%) patients in the non-NMV-r cohort (OR 0.5, CI 0.39-0.67; p<0.005) consistent with 45% relative risk reduction. A significant reduction in multisystem symptom burden and subsequent complications such as lower respiratory tract infection, cardiac arrhythmia, and diagnostic radiology testing were noted in NMV-r treated patients. There was no apparent increase serious complications between days 10 to 30.
Conclusion Treatment with NMV-r in non-hospitalized vaccinated patients with Covid-19 was associated with a reduced likelihood of emergency room visits, hospitalization, or death. Complications and overall resource utilization were also decreased.
References
Bernal, Da Silva, Musungaie, Molnupiravir for Oral Treatment of Covid-19 in Nonhospitalized Patients, N Engl J Med
Commissioner, The, Coronavirus (COVID-19) Update: FDA Authorizes New Monoclonal Antibody for Treatment of COVID-19 that Retains Activity Against Omicron Variant
Gottlieb, Vaca, Paredes, Early Remdesivir to Prevent Progression to Severe Covid-19 in Outpatients, N Engl J Med
Hammond, Leister-Tebbe, Gardner, Oral Nirmatrelvir for High-Risk, Nonhospitalized Adults with Covid-19, N Engl J Med
Mensah, Lacy, Stowe, Disease severity during SARS-COV-2 reinfection: a nationwide study, Journal of Infection
Paxlovid, added to PANORAMIC study -PANORAMIC
Pfizer, An Interventional Efficacy And Safety, Phase 2/3, Double-Blind, 2 Arm Study To Investigate Orally Administered Pf
Tenforde, Self, Adams, Association Between mRNA Vaccination and COVID-19 Hospitalization and Disease Severity, JAMA
Vanderweele, Ding, Sensitivity Analysis in Observational Research: Introducing the E-Value, Ann Intern Med
DOI record:
{
"DOI": "10.1093/cid/ciac673",
"ISSN": [
"1058-4838",
"1537-6591"
],
"URL": "http://dx.doi.org/10.1093/cid/ciac673",
"abstract": "<jats:title>Abstract</jats:title>\n <jats:sec>\n <jats:title>Background</jats:title>\n <jats:p>Treatment of coronavirus disease-2019 (Covid-19) with nirmatrelvir plus ritonavir (NMV-r) in high-risk non-hospitalized unvaccinated patients reduced the risk of progression to severe disease. However, the potential benefits of NMV-r among vaccinated patients are unclear.</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>Methods</jats:title>\n <jats:p>We conducted a comparative retrospective cohort study using the TriNetX research network. Patients ≥18 years of age who were vaccinated and subsequently developed Covid-19 between December 1, 2021, and April 18, 2022, were included. Cohorts were developed based on the use of NMV-r within five days of diagnosis. The primary composite outcome was all-cause emergency room (ER) visit, hospitalization, or death at a 30-days follow-up. Secondary outcomes included individual components of primary outcomes, multisystem symptoms, Covid-19 associated complications, and diagnostic test utilization.</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>Results</jats:title>\n <jats:p>After propensity score matching, 1,130 patients remained in each cohort. A primary composite outcome of all-cause ER visits, hospitalization, or death in 30 days occurred in 89 (7.87%) patients in the NMV-r cohort as compared to 163 (14.4%) patients in the non-NMV-r cohort (OR 0.5, CI 0.39-0.67; p&lt;0.005) consistent with 45% relative risk reduction. A significant reduction in multisystem symptom burden and subsequent complications such as lower respiratory tract infection, cardiac arrhythmia, and diagnostic radiology testing were noted in NMV-r treated patients. There was no apparent increase serious complications between days 10 to 30.</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>Conclusion</jats:title>\n <jats:p>Treatment with NMV-r in non-hospitalized vaccinated patients with Covid-19 was associated with a reduced likelihood of emergency room visits, hospitalization, or death. Complications and overall resource utilization were also decreased.</jats:p>\n </jats:sec>",
"author": [
{
"ORCID": "http://orcid.org/0000-0003-0296-6864",
"affiliation": [
{
"name": "Division of Cardiovascular Medicine, Department of Medicine, Lahey Hospital and Medical Center, Beth Israel Lahey Health , Burlington, MA , USA"
}
],
"authenticated-orcid": false,
"family": "Ganatra",
"given": "Sarju",
"sequence": "first"
},
{
"affiliation": [
{
"name": "Division of Cardiovascular Medicine, Department of Medicine, Lahey Hospital and Medical Center, Beth Israel Lahey Health , Burlington, MA , USA"
}
],
"family": "Dani",
"given": "Sourbha S",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Division of Cardiovascular Medicine, Department of Medicine, Lahey Hospital and Medical Center, Beth Israel Lahey Health , Burlington, MA , USA"
}
],
"family": "Ahmad",
"given": "Javaria",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Medicine, Cleveland Clinic Akron General , Akron, OH , USA"
}
],
"family": "Kumar",
"given": "Ashish",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Division of Cardiovascular Medicine, Department of Medicine, Lahey Hospital and Medical Center, Beth Israel Lahey Health , Burlington, MA , USA"
}
],
"family": "Shah",
"given": "Jui",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Division of Infectious Disease, Department of Medicine, Saint Vincent Hospital , Worcester, MA , USA"
}
],
"family": "Abraham",
"given": "George M",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Medicine, University of California San Francisco , San Francisco, CA , USA"
}
],
"family": "McQuillen",
"given": "Daniel P",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Medicine, University of California San Francisco , San Francisco, CA , USA"
}
],
"family": "Wachter",
"given": "Robert M",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Division of Infectious Disease, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School , Boston, MA , USA"
}
],
"family": "Sax",
"given": "Paul E",
"sequence": "additional"
}
],
"container-title": "Clinical Infectious Diseases",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2022,
8,
20
]
],
"date-time": "2022-08-20T08:57:39Z",
"timestamp": 1660985859000
},
"deposited": {
"date-parts": [
[
2022,
8,
20
]
],
"date-time": "2022-08-20T08:57:40Z",
"timestamp": 1660985860000
},
"indexed": {
"date-parts": [
[
2022,
8,
20
]
],
"date-time": "2022-08-20T09:13:54Z",
"timestamp": 1660986834800
},
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2022,
8,
20
]
]
},
"language": "en",
"license": [
{
"URL": "https://academic.oup.com/journals/pages/open_access/funder_policies/chorus/standard_publication_model",
"content-version": "am",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
8,
20
]
],
"date-time": "2022-08-20T00:00:00Z",
"timestamp": 1660953600000
}
}
],
"link": [
{
"URL": "https://academic.oup.com/cid/advance-article-pdf/doi/10.1093/cid/ciac673/45485775/ciac673.pdf",
"content-type": "application/pdf",
"content-version": "am",
"intended-application": "syndication"
},
{
"URL": "https://academic.oup.com/cid/advance-article-pdf/doi/10.1093/cid/ciac673/45485775/ciac673.pdf",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "286",
"original-title": [],
"prefix": "10.1093",
"published": {
"date-parts": [
[
2022,
8,
20
]
]
},
"published-online": {
"date-parts": [
[
2022,
8,
20
]
]
},
"publisher": "Oxford University Press (OUP)",
"reference-count": 0,
"references-count": 0,
"relation": {},
"resource": {
"primary": {
"URL": "https://academic.oup.com/cid/advance-article/doi/10.1093/cid/ciac673/6672670"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Infectious Diseases",
"Microbiology (medical)"
],
"subtitle": [],
"title": "Oral Nirmatrelvir and Ritonavir in Non-hospitalized Vaccinated Patients with Covid-19",
"type": "journal-article"
}