Molecular Mechanisms of Drug Resistance and Compensation in SARS-CoV-2 Main Protease: The Interplay Between E166 and L50
et al., bioRxiv, doi:10.1101/2025.01.24.634813, Jan 2025
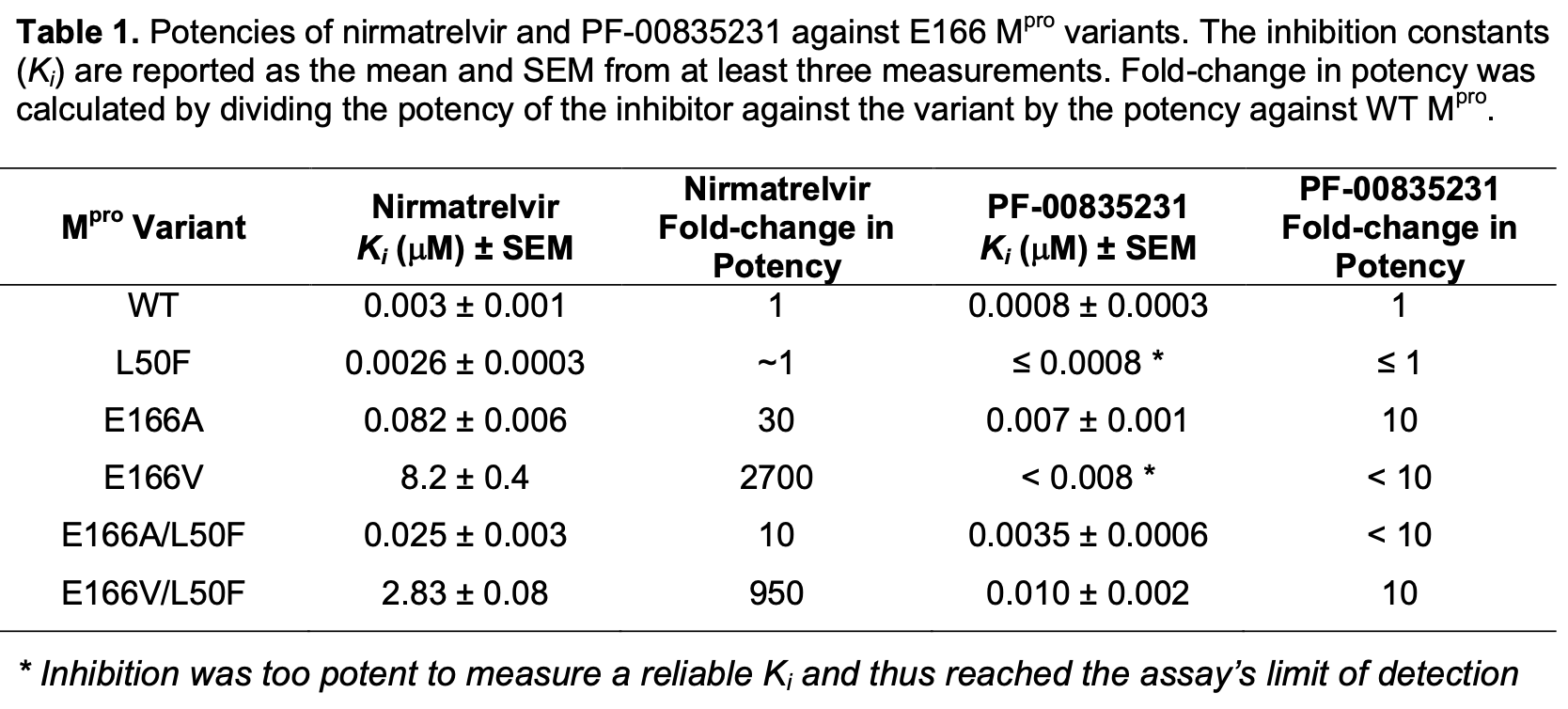
In vitro study showing that mutations at position E166 in the SARS-CoV-2 main protease (Mpro) confer resistance to nirmatrelvir, the active component of paxlovid, while preserving substrate cleavage. Authors found that E166A and E166V mutations reduced nirmatrelvir potency by up to 3000-fold but only reduced catalytic efficiency by up to 2-fold. Addition of the L50F mutation compensated for this catalytic deficiency in the E166 mutants. Cocrystal structures revealed that E166 is critical for dimerization and shaping the S1 binding pocket. The findings highlight the mutability of E166 as a prime site for resistance mutations and the potential for compensatory mutations like L50F to enable emergence of highly resistant but still active Mpro variants.
Zvornicanin et al., 27 Jan 2025, USA, preprint, 10 authors.
Contact: celia.schiffer@umassmed.edu (corresponding author).
In vitro studies are an important part of preclinical research, however results may be very different in vivo.
Molecular Mechanisms of Drug Resistance and Compensation in SARS-CoV-2 Main Protease: The Interplay Between E166 and L50
doi:10.1101/2025.01.24.634813
The SARS-CoV-2 main protease (M pro ) is essential for viral replication, and a primary target for COVID-19 antivirals. Direct-acting antivirals such as nirmatrelvir, the active component of Paxlovid, target the M pro active site to block viral polyprotein cleavage and thus replication. However, drug resistance mutations at the active site residue Glu166 (E166) have emerged in in vitro selection studies, raising concerns about the durability of current antiviral strategies. Here, we investigate the molecular basis of drug resistance conferred by E166A and E166V mutations against nirmatrelvir and the related PF-00835231, individually and in combination with the distal mutation L50F. We found that E166 mutations reduce nirmatrelvir potency by up to 3000-fold while preserving substrate cleavage, with catalytic efficiency reduced by only up to 2fold. This loss of catalytic efficiency was compensated for by the addition of L50F in the doublemutant variants. We have determined three cocrystal structures of the E166 variants (E166A, E166V, and E166V/L50F) bound to PF-00835231. Comparison of these structures with wildtype demonstrated that E166 is crucial for dimerization and for shaping the substrate-binding S1 pocket. Our findings highlight the mutability of E166, a prime site for resistance for inhibitors that leverage direct interactions with this position, and the potential emergence of highly resistant and active variants in combination with the compensatory mutation L50F. These insights support the design of inhibitors that target conserved protease features and avoid E166 sidechain interactions to minimize susceptibility to resistance.
Enzyme inhibition assays Inhibition assays were performed in the same assay buffer (50 mM Tris pH 7.5, 50 mM NaCl, 1 mM ethylenediaminetetraacetic acid (EDTA), 1 mM dithiothreitol (DTT), but with only 1% DMSO. To determine the inhibition constant K i , enzyme was incubated at room temperature with increasing concentrations of nirmatrelvir or PF-00835231 for 1 hour in assay buffer. The enzymatic reaction was initiated with 40 μM protease FRET substrate and monitored using a PerkinElmer Envision plate reader. At least three replicates were performed for each inhibitor concentration. The initial velocity for each reaction was calculated by linear regression. The K i was calculated by plotting the initial velocity (RFU/s) at each inhibitor concentration (µM) and then fit to the Morrison equation, using each enzyme's respective K M , in GraphPad Prism 10 28 software. Enzyme levels were adjusted according to the K i estimates from initial experiments to maintain [E]/K i <100 to ensure confidence in K i determinations. In cases of very potent inhibition where the enzyme concentration could not be decreased further due to loss of signal, [E]/100 is reported as the upper estimate for K i as the assay detection limit.
Native Mass Spectrometry Each protein was purified in SEC buffer (25 mM HEPES pH 7.5, 150 mM NaCl, and 1 mM TCEP) and then diluted to 2-4 mg/mL in 50-100 µL in the same buffer. The protein was dialyzed for three hours into 200 mM ammonium acetate (pH 6.8) using a..
References
Adams, Afonine, Bunkóczi, Chen, Davis et al., PHENIX: A Comprehensive Python-Based System for Macromolecular Structure Solution, Acta Crystallogr D Biol Crystallogr, doi:10.1107/S0907444909052925.(27
Boras, Jones, Anson, Arenson, Aschenbrenner et al., Preclinical Characterization of an Intravenous Coronavirus 3CL Protease Inhibitor for the Potential Treatment of COVID19, Nat Commun, doi:10.1038/s41467-021-26239-2
Brünger, Free R Value: A Novel Statistical Quantity for Assessing the Accuracy of Crystal Structures, Nature, doi:10.1038/355472a0
Duan, Zhou, Liu, Iketani, Lin et al., Molecular Mechanisms of SARS-CoV-2 Resistance to Nirmatrelvir, Nature, doi:10.1038/s41586-023-06609-0
El-Baba, Lutomski, Kantsadi, Malla, John et al., Allosteric Inhibition of the SARS-CoV-2 Main Protease: Insights from Mass Spectrometry Based Assays*, Angew Chem Int Ed Engl, doi:10.1002/anie.202010316
Emsley, Cowtan, Coot: Model-Building Tools for Molecular Graphics, Acta Crystallogr D Biol Crystallogr, doi:10.1107/S0907444904019158
Flynn, Huang, Zvornicanin, Schneider-Nachum, Shaqra et al., Systematic Analyses of the Resistance Potential of Drugs Targeting SARS-CoV-2 Main Protease, ACS Infect Dis, doi:10.1021/acsinfecdis.3c00125
Flynn, Samant, Schneider-Nachum, Barkan, Yilmaz et al., Comprehensive Fitness Landscape of SARS-CoV-2 Mpro Reveals Insights into Viral Resistance Mechanisms, Elife, doi:10.7554/eLife.77433
Flynn, Zvornicanin, Tsepal, Shaqra, Kurt Yilmaz et al., Contributions of Hyperactive Mutations in Mpro from SARS-CoV-2 to Drug Resistance, ACS Infect Dis, doi:10.1021/acsinfecdis.3c00560
Gammeltoft, Zhou, Ryberg, Pham, Binderup et al., Substitutions in SARS-CoV-2 Mpro Selected by Protease Inhibitor Boceprevir Confer Resistance to Nirmatrelvir, Viruses, doi:10.3390/v15091970
Havranek, Demissie, Lee, Lan, Zhang et al., Discovery of Nirmatrelvir Resistance Mutations in SARS-CoV-2 3CLpro: A Computational-Experimental Approach, J Chem Inf Model, doi:10.1021/acs.jcim.3c01269
Henes, Lockbaum, Kosovrasti, Leidner, Nachum et al., Picomolar to Micromolar: Elucidating the Role of Distal Mutations in HIV-1 Protease in Conferring Drug Resistance, ACS Chem Biol, doi:10.1021/acschembio.9b00370
Hoffman, Kania, Brothers, Davies, Ferre et al., Discovery of Ketone-Based Covalent Inhibitors of Coronavirus 3CL Proteases for the Potential Therapeutic Treatment of COVID-19, J Med Chem, doi:10.1021/acs.jmedchem.0c01063
Hu, Lewandowski, Tan, Zhang, Morgan et al., Naturally Occurring Mutations of SARS-CoV-2 Main Protease Confer Drug Resistance to Nirmatrelvir, ACS Cent. Sci, doi:10.1021/acscentsci.3c00538
Iketani, Mohri, Culbertson, Hong, Duan et al., Multiple Pathways for SARS-CoV-2 Resistance to Nirmatrelvir, Nature, doi:10.1038/s41586-022-05514-2
Jochmans, Liu, Donckers, Stoycheva, Boland et al., The Substitutions L50F, E166A, and L167F in SARS-CoV-2 3CLpro Are Selected by a Protease Inhibitor In Vitro and Confer Resistance To Nirmatrelvir, mBio, doi:10.1128/mbio.02815-22
Kabsch, Xds, None, Acta Crystallogr D Biol Crystallogr, doi:10.1107/S0907444909047337
Mccoy, Grosse-Kunstleve, Adams, Winn, Storoni et al., Phaser Crystallographic Software, J Appl Crystallogr, doi:10.1107/S0021889807021206
Noske, De Souza Silva, De Godoy, Dolci, Fernandes et al., Structural Basis of Nirmatrelvir and Ensitrelvir Activity against Naturally Occurring Polymorphisms of the SARS-CoV-2 Main Protease, J Biol Chem, doi:10.1016/j.jbc.2023.103004
Padhi, Tripathi, Hotspot Residues and Resistance Mutations in the Nirmatrelvir-Binding Site of SARS-CoV-2 Main Protease: Design, Identification, and Correlation with Globally Circulating Viral Genomes, Biochem Biophys Res Commun, doi:10.1016/j.bbrc.2022.09.010
Shaqra, Zvornicanin, Huang, Lockbaum, Knapp et al., Defining the Substrate Envelope of SARS-CoV-2 Main Protease to Predict and Avoid Drug Resistance, Nat Commun, doi:10.1038/s41467-022-31210-w
Silvestrini, Belhaj, Comez, Gerelli, Lauria et al., The Dimer-Monomer Equilibrium of SARS-CoV-2 Main Protease Is Affected by Small Molecule Inhibitors, Sci Rep, doi:10.1038/s41598-021-88630-9
Zhang, Xie, Luo, Qian, Yang et al., Resistance Mechanisms of SARS-CoV-2 3CLpro to the Non-Covalent Inhibitor WU-04, Cell Discov, doi:10.1038/s41421-024-00673-0
Zhou, Gammeltoft, Ryberg, Pham, Tjørnelund et al., Nirmatrelvir-Resistant SARS-CoV-2 Variants with High Fitness in an Infectious Cell Culture System, Sci Adv, doi:10.1126/sciadv.add7197
Zhu, Yurgelonis, Noell, Yang, Guan et al., In Vitro Selection and Analysis of SARS-CoV-2 Nirmatrelvir Resistance Mutations Contributing to Clinical Virus Resistance Surveillance, Sci. Adv, doi:10.1126/sciadv.adl4013
Zvornicanin, Shaqra, Huang, Ornelas, Moghe et al., Crystal Structures of Inhibitor-Bound Main Protease from Delta-and Gamma-Coronaviruses, Viruses, doi:10.3390/v15030781
DOI record:
{
"DOI": "10.1101/2025.01.24.634813",
"URL": "http://dx.doi.org/10.1101/2025.01.24.634813",
"abstract": "<jats:title>Abstract</jats:title><jats:p>The SARS-CoV-2 main protease (M<jats:sup>pro</jats:sup>) is essential for viral replication, and a primary target for COVID-19 antivirals. Direct-acting antivirals such as nirmatrelvir, the active component of Paxlovid, target the M<jats:sup>pro</jats:sup>active site to block viral polyprotein cleavage and thus replication. However, drug resistance mutations at the active site residue Glu166 (E166) have emerged in<jats:italic>in vitro</jats:italic>selection studies, raising concerns about the durability of current antiviral strategies. Here, we investigate the molecular basis of drug resistance conferred by E166A and E166V mutations against nirmatrelvir and the related PF-00835231, individually and in combination with the distal mutation L50F. We found that E166 mutations reduce nirmatrelvir potency by up to 3000-fold while preserving substrate cleavage, with catalytic efficiency reduced by only up to 2- fold. This loss of catalytic efficiency was compensated for by the addition of L50F in the double- mutant variants. We have determined three cocrystal structures of the E166 variants (E166A, E166V, and E166V/L50F) bound to PF-00835231. Comparison of these structures with wild- type demonstrated that E166 is crucial for dimerization and for shaping the substrate-binding S1 pocket. Our findings highlight the mutability of E166, a prime site for resistance for inhibitors that leverage direct interactions with this position, and the potential emergence of highly resistant and active variants in combination with the compensatory mutation L50F. These insights support the design of inhibitors that target conserved protease features and avoid E166 side- chain interactions to minimize susceptibility to resistance.</jats:p>",
"accepted": {
"date-parts": [
[
2025,
1,
27
]
]
},
"author": [
{
"affiliation": [],
"family": "Zvornicanin",
"given": "Sarah N.",
"sequence": "first"
},
{
"affiliation": [],
"family": "Shaqra",
"given": "Ala M.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Flynn",
"given": "Julia",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Carias Martinez",
"given": "Heidi",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Jia",
"given": "Weiping",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Moquin",
"given": "Stephanie",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Dovala",
"given": "Dustin",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0001-5857-6676",
"affiliation": [],
"authenticated-orcid": false,
"family": "Bolon",
"given": "Daniel N.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kurt Yilmaz",
"given": "Nese",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Schiffer",
"given": "Celia A.",
"sequence": "additional"
}
],
"container-title": [],
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2025,
1,
28
]
],
"date-time": "2025-01-28T03:45:13Z",
"timestamp": 1738035913000
},
"deposited": {
"date-parts": [
[
2025,
1,
30
]
],
"date-time": "2025-01-30T12:35:14Z",
"timestamp": 1738240514000
},
"group-title": "Biochemistry",
"indexed": {
"date-parts": [
[
2025,
1,
30
]
],
"date-time": "2025-01-30T13:10:16Z",
"timestamp": 1738242616151,
"version": "3.34.0"
},
"institution": [
{
"name": "bioRxiv"
}
],
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2025,
1,
27
]
]
},
"license": [
{
"URL": "http://creativecommons.org/licenses/by-nc-nd/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2025,
1,
27
]
],
"date-time": "2025-01-27T00:00:00Z",
"timestamp": 1737936000000
}
}
],
"link": [
{
"URL": "https://syndication.highwire.org/content/doi/10.1101/2025.01.24.634813",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "246",
"original-title": [],
"posted": {
"date-parts": [
[
2025,
1,
27
]
]
},
"prefix": "10.1101",
"published": {
"date-parts": [
[
2025,
1,
27
]
]
},
"publisher": "Cold Spring Harbor Laboratory",
"reference": [
{
"DOI": "10.1038/s41467-021-26239-2",
"doi-asserted-by": "publisher",
"key": "2025013004350575000_2025.01.24.634813v1.1"
},
{
"DOI": "10.1021/acs.jmedchem.0c01063",
"doi-asserted-by": "publisher",
"key": "2025013004350575000_2025.01.24.634813v1.2"
},
{
"DOI": "10.1126/sciadv.add7197",
"doi-asserted-by": "publisher",
"key": "2025013004350575000_2025.01.24.634813v1.3"
},
{
"DOI": "10.1038/s41586-022-05514-2",
"doi-asserted-by": "publisher",
"key": "2025013004350575000_2025.01.24.634813v1.4"
},
{
"DOI": "10.1126/sciadv.adl4013",
"doi-asserted-by": "publisher",
"key": "2025013004350575000_2025.01.24.634813v1.5"
},
{
"DOI": "10.1021/acscentsci.3c00538",
"doi-asserted-by": "publisher",
"key": "2025013004350575000_2025.01.24.634813v1.6"
},
{
"DOI": "10.1016/j.bbrc.2022.09.010",
"doi-asserted-by": "publisher",
"key": "2025013004350575000_2025.01.24.634813v1.7"
},
{
"DOI": "10.7554/eLife.77433",
"doi-asserted-by": "publisher",
"key": "2025013004350575000_2025.01.24.634813v1.8"
},
{
"DOI": "10.1021/acsinfecdis.3c00125",
"doi-asserted-by": "publisher",
"key": "2025013004350575000_2025.01.24.634813v1.9"
},
{
"DOI": "10.1128/mbio.02815-22",
"doi-asserted-by": "publisher",
"key": "2025013004350575000_2025.01.24.634813v1.10"
},
{
"DOI": "10.3390/v15091970",
"doi-asserted-by": "publisher",
"key": "2025013004350575000_2025.01.24.634813v1.11"
},
{
"DOI": "10.1016/j.jbc.2023.103004",
"doi-asserted-by": "publisher",
"key": "2025013004350575000_2025.01.24.634813v1.12"
},
{
"DOI": "10.1038/s41586-023-06609-0",
"doi-asserted-by": "publisher",
"key": "2025013004350575000_2025.01.24.634813v1.13"
},
{
"DOI": "10.1021/acs.jcim.3c01269",
"doi-asserted-by": "publisher",
"key": "2025013004350575000_2025.01.24.634813v1.14"
},
{
"DOI": "10.1021/acschembio.9b00370",
"doi-asserted-by": "publisher",
"key": "2025013004350575000_2025.01.24.634813v1.15"
},
{
"DOI": "10.1021/acsinfecdis.3c00560",
"doi-asserted-by": "publisher",
"key": "2025013004350575000_2025.01.24.634813v1.16"
},
{
"DOI": "10.1038/s41421-024-00673-0",
"doi-asserted-by": "publisher",
"key": "2025013004350575000_2025.01.24.634813v1.17"
},
{
"DOI": "10.1038/s41598-021-88630-9",
"doi-asserted-by": "publisher",
"key": "2025013004350575000_2025.01.24.634813v1.18"
},
{
"DOI": "10.1002/anie.202010316",
"doi-asserted-by": "publisher",
"key": "2025013004350575000_2025.01.24.634813v1.19"
},
{
"DOI": "10.1038/s41467-022-31210-w",
"doi-asserted-by": "publisher",
"key": "2025013004350575000_2025.01.24.634813v1.20"
},
{
"DOI": "10.3390/v15030781",
"doi-asserted-by": "publisher",
"key": "2025013004350575000_2025.01.24.634813v1.21"
},
{
"DOI": "10.1107/S0907444909047337",
"doi-asserted-by": "publisher",
"key": "2025013004350575000_2025.01.24.634813v1.22"
},
{
"DOI": "10.1107/S0021889807021206",
"doi-asserted-by": "publisher",
"key": "2025013004350575000_2025.01.24.634813v1.23"
},
{
"DOI": "10.1038/355472a0",
"doi-asserted-by": "publisher",
"key": "2025013004350575000_2025.01.24.634813v1.24"
},
{
"DOI": "10.1107/S0907444904019158",
"doi-asserted-by": "publisher",
"key": "2025013004350575000_2025.01.24.634813v1.25"
},
{
"DOI": "10.1107/S0907444909052925",
"doi-asserted-by": "publisher",
"key": "2025013004350575000_2025.01.24.634813v1.26"
},
{
"key": "2025013004350575000_2025.01.24.634813v1.27",
"unstructured": "The PyMOL Molecular Graphics System."
}
],
"reference-count": 27,
"references-count": 27,
"relation": {},
"resource": {
"primary": {
"URL": "http://biorxiv.org/lookup/doi/10.1101/2025.01.24.634813"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"subtype": "preprint",
"title": "Molecular Mechanisms of Drug Resistance and Compensation in SARS-CoV-2 Main Protease: The Interplay Between E166 and L50",
"type": "posted-content"
}