Predictors of nirmatrelvir–ritonavir receipt among COVID-19 patients in a large US health system
et al., Scientific Reports, doi:10.1038/s41598-024-57633-7, Mar 2024
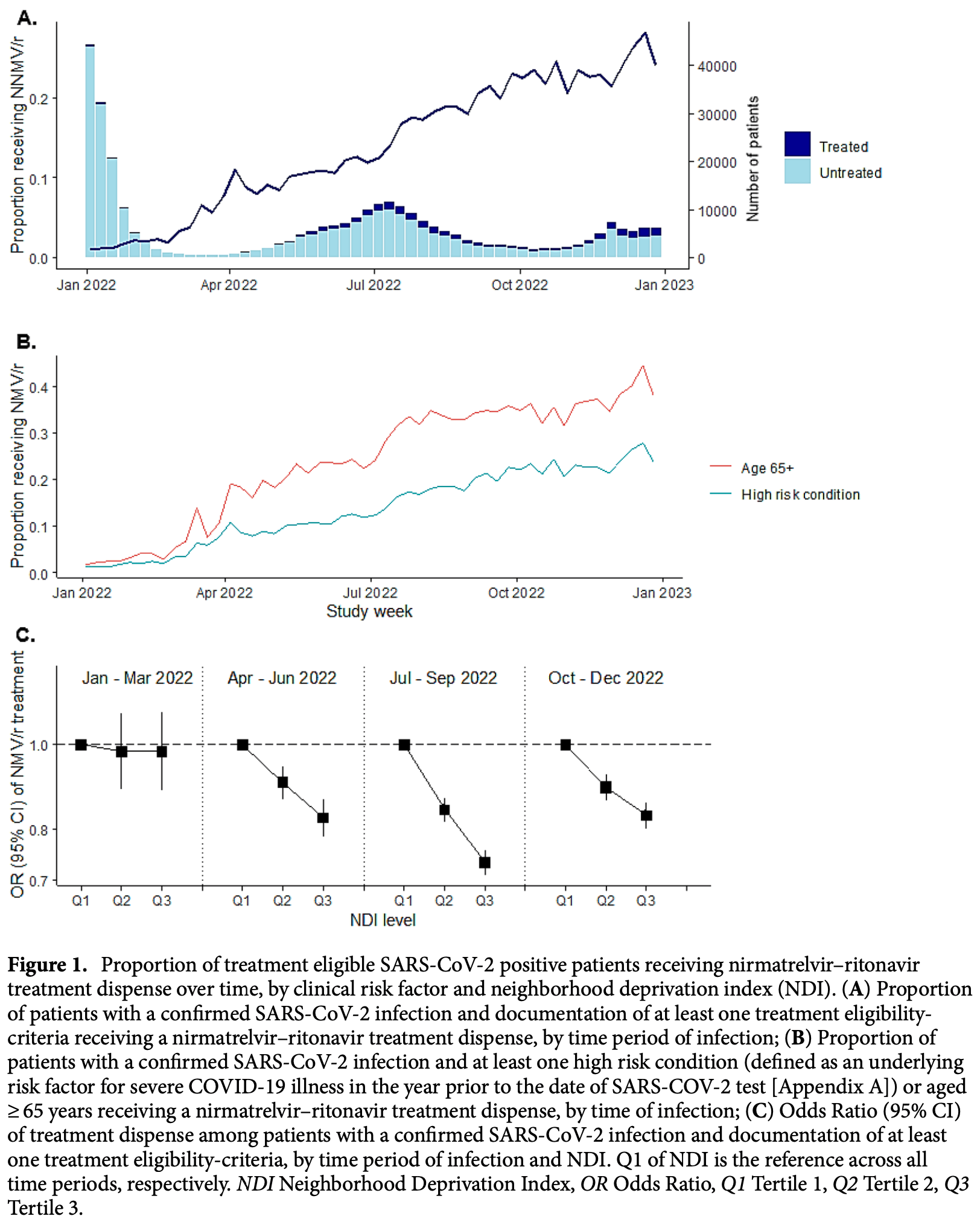
Retrospective 319,900 treatment-eligible COVID-19 patients showing relatively low use of paxlovid and significant socioeconomic disparities. Treated patients were more likely to be from affluent neighborhoods, be up to date on vaccinations, and have more documented health conditions. These factors suggest that treated patients may be more health-conscious and have better access to care in general. Most retrospective studies do not adequately control for this confounding, leading to significant bias and overestimation of effectiveness.
Resistance. Variants may be resistant to paxlovid1-8. Use may promote the emergence of variants that weaken host immunity and potentially contribute to long COVID9. Confounding by contraindication. Hoertel et al. find that over 50% of patients that died had a contraindication for the use of Paxlovid10. Retrospective studies that do not exclude contraindicated patients may significantly overestimate efficacy. Black box warning. The FDA notes that severe, life-threatening, and/or fatal adverse reactions due to drug interactions have been reported in patients treated with paxlovid11. Kidney and liver injury. Studies show significantly increased risk of acute kidney injury12 and liver injury13,14. Viral rebound. Studies show significantly increased risk of replication-competent viral rebound15-17.
1.
Zhou et al., Nirmatrelvir-resistant SARS-CoV-2 variants with high fitness in an infectious cell culture system, Science Advances, doi:10.1126/sciadv.add7197.
2.
Moghadasi et al., Rapid resistance profiling of SARS-CoV-2 protease inhibitors, npj Antimicrobials and Resistance, doi:10.1038/s44259-023-00009-0.
3.
Jochmans et al., The Substitutions L50F, E166A, and L167F in SARS-CoV-2 3CLpro Are Selected by a Protease Inhibitor In Vitro and Confer Resistance To Nirmatrelvir, mBio, doi:10.1128/mbio.02815-22.
4.
Lopez et al., SARS-CoV-2 Resistance to Small Molecule Inhibitors, Current Clinical Microbiology Reports, doi:10.1007/s40588-024-00229-6.
5.
Zvornicanin et al., Molecular Mechanisms of Drug Resistance and Compensation in SARS-CoV-2 Main Protease: The Interplay Between E166 and L50, bioRxiv, doi:10.1101/2025.01.24.634813.
6.
Vukovikj et al., Impact of SARS-CoV-2 variant mutations on susceptibility to monoclonal antibodies and antiviral drugs: a non-systematic review, April 2022 to October 2024, Eurosurveillance, doi:10.2807/1560-7917.ES.2025.30.10.2400252.
7.
Deschenes et al., Functional and structural characterization of treatment-emergent nirmatrelvir resistance mutations at low frequencies in the main protease (Mpro) reveals a unique evolutionary route for SARS-CoV-2 to gain resistance, The Journal of Infectious Diseases, doi:10.1093/infdis/jiaf294.
8.
Zhou (B) et al., SARS-CoV-2 Mpro inhibitor ensitrelvir: asymmetrical cross-resistance with nirmatrelvir and emerging resistance hotspots, Emerging Microbes & Infections, doi:10.1080/22221751.2025.2552716.
9.
Thomas et al., Nirmatrelvir-Resistant Mutations in SARS-CoV-2 Mpro Enhance Host Immune Evasion via Cleavage of NF-κB Essential Modulator, bioRxiv, doi:10.1101/2024.10.18.619137.
10.
Hoertel et al., Prevalence of Contraindications to Nirmatrelvir-Ritonavir Among Hospitalized Patients With COVID-19 at Risk for Progression to Severe Disease, JAMA Network Open, doi:10.1001/jamanetworkopen.2022.42140.
11.
FDA, Fact sheet for healthcare providers: emergency use authorization for paxlovid, www.fda.gov/media/155050/download.
12.
Kamo et al., Association of Antiviral Drugs for the Treatment of COVID-19 With Acute Renal Failure, In Vivo, doi:10.21873/invivo.13637.
13.
Wang et al., Development and validation of a nomogram to assess the occurrence of liver dysfunction in patients with COVID-19 pneumonia in the ICU, BMC Infectious Diseases, doi:10.1186/s12879-025-10684-1.
14.
Siby et al., Temporal Trends in Serious Adverse Events Associated with Oral Antivirals During the COVID-19 Pandemic: Insights from the FAERS Database (2020–2023), Open Forum Infectious Diseases, doi:10.1093/ofid/ofaf695.1825.
15.
Edelstein et al., SARS-CoV-2 virologic rebound with nirmatrelvir-ritonavir therapy, medRxiv, doi:10.1101/2023.06.23.23288598.
Malden et al., 29 Mar 2024, retrospective, USA, peer-reviewed, survey, mean age 47.8, 11 authors, study period 1 January, 2022 - 31 December, 2022.
Contact: sara.y.tartof@kp.org.
Predictors of nirmatrelvir–ritonavir receipt among COVID-19 patients in a large US health system
Scientific Reports, doi:10.1038/s41598-024-57633-7
A clear understanding of real-world uptake of nirmatrelvir-ritonavir for treatment of SARS-CoV-2 can inform treatment allocation strategies and improve interpretation of effectiveness studies. We used data from a large US healthcare system to describe nirmatrelvir-ritonavir dispenses among all SARS-CoV-2 positive patients aged ≥ 12 years meeting recommended National Institutes of Health treatment eligibility criteria for the study period between 1 January and 31 December, 2022. Overall, 10.9% (N = 34,791/319,900) of treatment eligible patients with SARS-CoV-2 infections received nirmatrelvir-ritonavir over the study period. Although uptake of nirmatrelvir-ritonavir increased over time, by the end of 2022, less than a quarter of treatment eligible patients with SARS-CoV-2 infections had received nirmatrelvir-ritonavir. Across patient demographics, treatment was generally consistent with tiered treatment guidelines, with dispenses concentrated among patients aged ≥ 65 years (14,706/63,921; 23.0%), and with multiple comorbidities (10,989/54,431; 20.1%). However, neighborhoods of lower socioeconomic status (upper third of neighborhood deprivation index [NDI]) had between 12% (95% CI: 7-18%) and 28% (25-32%) lower odds of treatment dispense over the time periods studied compared to the lower third of NDI distribution, even after accounting for demographic and clinical characteristics. A limited chart review (N = 40) confirmed that in some cases a decision not to treat was appropriate and aligned with national guidelines to use clinical judgement on a case-by-case basis. There is a need to enhance patient and provider awareness on the availability and benefits of nirmatrelvir-ritonavir for the treatment of COVID-19 illness. Nirmatrelvir is an oral antiviral that, when co-administered with ritonavir within 5 days of symptom onset, is highly effective at reducing the risk of hospitalization and death among patients with mild-to-moderate COVID-19 who are at risk for progression to severe disease 1-6 . Accordingly, nirmatrelvir-ritonavir received Emergency Use Authorization (EUA) from the Food and Drug Administration (FDA) in December 2021. Nirmatrelvir-ritonavir treatment eligibility depends on the presence of underlying risk factors for progression to severe COVID-19 including age and vaccination status, weight, renal and hepatic function, and current use of select medications known to interact with nirmatrelvir-ritonavir. In the United States, initial guidelines recommended a tiered prioritization approach to treatment based on clinical risk 7 . As knowledge and treatment availability expanded throughout 2022, recommendations adapted to widen treatment eligibility criteria, eventually including all persons aged 65 years and older or aged 12 years and older with one or more clinical risk factors for progression to severe COVID-19. More recently (i.e., after our study period), treatment eligibility criteria have expanded further to include all adults aged..
Author contributions DM constructed the analysis plan, assisted with the analysis and drafted the manuscript. DM, SYT, JAL, BKA, LP, VH, JS and JMM designed the project. VH completed the programming and data analysis. All authors revised the manuscript and provided critical input.
Competing interests JAL has received grants and consultancy fees from Pfizer. SYT and TF have received grants from Pfizer paid directly to their institution. LP and JMM are employees of Pfizer and hold stock and stock options in Pfizer. BKA receives research support from Dynavax, Moderna, GlaxoSmithKline, Pfizer and Genentech, for projects outside of the submitted work. JS has received grants from Pfizer, ALK Inc., Novavax and Dynavax Technologies paid directly to his institution. DM, VH and JK have no conflicts of interest to declare. This study was funded by Pfizer.
References
Aggarwal, Real-world use of nirmatrelvir-ritonavir in outpatients with COVID-19 during the era of omicron variants including BA.4 and BA.5 in Colorado, USA: A retrospective cohort study, Lancet Infect. Dis
Appaneal, Nirmatrelvir/ritonavir utilization for the treatment of non-hospitalized adults with COVID-19 in the National Veterans Affairs (VA) Healthcare System, Infect. Dis. Ther
Arbel, Nirmatrelvir use and severe Covid-19 outcomes during the omicron surge, N. Engl. J. Med
Betsch, Wieler, Habersaat, Monitoring behavioural insights related to COVID-19, Lancet Public Health
Boehmer, Racial and ethnic disparities in outpatient treatment of COVID-19-United States, January-July 2022, MMWR Morb. Mortal. Wkly. Rep
Callaghan, Correlates and disparities of intention to vaccinate against COVID-19, Soc. Sci. Med
Davis, Comparing Kaiser Permanente members to the general population: Implications for generalizability of research, Perm. J
Dorn, Cooney, Sabin, COVID-19 exacerbating inequalities in the US, Lancet
Dryden-Peterson, Nirmatrelvir plus ritonavir for early COVID-19 and hospitalization in a large US health system, Ann. Intern. Med
Fda, PAXLOVID Patient Eligibility Screening Checklist Tool for Prescribers
Floyd, COVID-19 vaccination and mask wearing behaviors in the United States, August 2020-June 2021, Expert Rev. Vaccines
Ganatra, Oral nirmatrelvir and ritonavir in non-hospitalized vaccinated patients with Covid-19, Clin. Infect. Dis
Glasheen, Charlson comorbidity index: ICD-9 update and ICD-10 translation, Am. Health Drug Benefits
Gold, Dispensing of oral antiviral drugs for treatment of COVID-19 by zip code-level social vulnerability-United States, December 23, 2021, MMWR Morb. Mortal. Wkly. Rep
Hammond, Oral nirmatrelvir for high-risk, nonhospitalized adults with Covid-19, N. Engl. J. Med
Jefferson, Differences in COVID-19 testing and adverse outcomes by race, ethnicity, sex, and health system setting in a large diverse US cohort, PLoS One
Lewnard, Effectiveness of nirmatrelvir-ritonavir in preventing hospital admissions and deaths in people with COVID-19: A cohort study in a large US health-care system, Lancet Infect. Dis
Lindholt, Jørgensen, Bor, Petersen, Public acceptance of COVID-19 vaccines: Cross-national evidence on levels and individual-level predictors using observational data, BMJ Open
Magesh, Disparities in COVID-19 outcomes by race, ethnicity, and socioeconomic status, JAMA Netw. Open
Malden, Natural language processing for improved characterization of COVID-19 symptoms: Observational study of 350,000 patients in a large integrated health care system, JMIR Public Health Surveill
Manciulli, Safety and efficacy of outpatient treatments for COVID-19: Real-life data from a regionwide cohort of high-risk patients in Tuscany, Italy (the FEDERATE Cohort), Viruses
Mclaughlin, County-level predictors of coronavirus disease 2019 (COVID-19) cases and deaths in the United States: What happened, and where do we go from here?, Clin. Infect. Dis
Messer, The development of a standardized neighborhood deprivation index, J. Urban Health
Murphy, Samson, Sommers, COVID-19 Antivirals Utilization: Geographic and Demographic Patterns of Treatment in
Najjar-Debbiny, Effectiveness of paxlovid in reducing severe coronavirus disease 2019 and mortality in high-risk patients, Clin. Infect. Dis
O'hayer, Evolution of care and outcomes across surges in hospitalized patients with coronavirus disease 2019, Am. J. Med
Shah, Paxlovid associated with decreased hospitalization rate among adults with COVID-19-United States, April-September 2022, Am. J. Transplant
Sullivan, Notes from the field: Dispensing of oral antiviral drugs for treatment of COVID-19 by zip code-level social vulnerability-United States, December 23, 2021, MMWR Morb. Mortal. Wkly. Rep
Wong, Real-world effectiveness of molnupiravir and nirmatrelvir plus ritonavir against mortality, hospitalisation, and in-hospital outcomes among community-dwelling, ambulatory patients with confirmed SARS-CoV-2 infection during the omicron wave in Hong Kong: An observational study, Lancet
Yip, Impact of the use of oral antiviral agents on the risk of hospitalization in community COVID-19 patients, Clin. Infect. Dis
DOI record:
{
"DOI": "10.1038/s41598-024-57633-7",
"ISSN": [
"2045-2322"
],
"URL": "http://dx.doi.org/10.1038/s41598-024-57633-7",
"abstract": "<jats:title>Abstract</jats:title><jats:p>A clear understanding of real-world uptake of nirmatrelvir–ritonavir for treatment of SARS-CoV-2 can inform treatment allocation strategies and improve interpretation of effectiveness studies. We used data from a large US healthcare system to describe nirmatrelvir–ritonavir dispenses among all SARS-CoV-2 positive patients aged ≥ 12 years meeting recommended National Institutes of Health treatment eligibility criteria for the study period between 1 January and 31 December, 2022. Overall, 10.9% (N = 34,791/319,900) of treatment eligible patients with SARS-CoV-2 infections received nirmatrelvir–ritonavir over the study period. Although uptake of nirmatrelvir–ritonavir increased over time, by the end of 2022, less than a quarter of treatment eligible patients with SARS-CoV-2 infections had received nirmatrelvir–ritonavir. Across patient demographics, treatment was generally consistent with tiered treatment guidelines, with dispenses concentrated among patients aged ≥ 65 years (14,706/63,921; 23.0%), and with multiple comorbidities (10,989/54,431; 20.1%). However, neighborhoods of lower socioeconomic status (upper third of neighborhood deprivation index [NDI]) had between 12% (95% CI: 7–18%) and 28% (25–32%) lower odds of treatment dispense over the time periods studied compared to the lower third of NDI distribution, even after accounting for demographic and clinical characteristics. A limited chart review (N = 40) confirmed that in some cases a decision not to treat was appropriate and aligned with national guidelines to use clinical judgement on a case-by-case basis. There is a need to enhance patient and provider awareness on the availability and benefits of nirmatrelvir–ritonavir for the treatment of COVID-19 illness.</jats:p>",
"alternative-id": [
"57633"
],
"article-number": "7485",
"assertion": [
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Received",
"name": "received",
"order": 1,
"value": "27 July 2023"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Accepted",
"name": "accepted",
"order": 2,
"value": "20 March 2024"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "First Online",
"name": "first_online",
"order": 3,
"value": "29 March 2024"
},
{
"group": {
"label": "Competing interests",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 1,
"value": "JAL has received grants and consultancy fees from Pfizer. SYT and TF have received grants from Pfizer paid directly to their institution. LP and JMM are employees of Pfizer and hold stock and stock options in Pfizer. BKA receives research support from Dynavax, Moderna, GlaxoSmithKline, Pfizer and Genentech, for projects outside of the submitted work. JS has received grants from Pfizer, ALK Inc., Novavax and Dynavax Technologies paid directly to his institution. DM, VH and JK have no conflicts of interest to declare. This study was funded by Pfizer."
}
],
"author": [
{
"affiliation": [],
"family": "Malden",
"given": "Deborah E.",
"sequence": "first"
},
{
"affiliation": [],
"family": "McLaughlin",
"given": "John M.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hong",
"given": "Vennis",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lewnard",
"given": "Joseph",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ackerson",
"given": "Bradley K.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Puzniak",
"given": "Laura",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kim",
"given": "Jeniffer S.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Takhar",
"given": "Harpreet",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Frankland",
"given": "Timothy B.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Slezak",
"given": "Jeff M.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Tartof",
"given": "Sara Y.",
"sequence": "additional"
}
],
"container-title": "Scientific Reports",
"container-title-short": "Sci Rep",
"content-domain": {
"crossmark-restriction": false,
"domain": [
"link.springer.com"
]
},
"created": {
"date-parts": [
[
2024,
3,
29
]
],
"date-time": "2024-03-29T10:01:53Z",
"timestamp": 1711706513000
},
"deposited": {
"date-parts": [
[
2024,
3,
29
]
],
"date-time": "2024-03-29T10:03:34Z",
"timestamp": 1711706614000
},
"funder": [
{
"DOI": "10.13039/100004319",
"doi-asserted-by": "publisher",
"name": "Pfizer"
}
],
"indexed": {
"date-parts": [
[
2024,
3,
30
]
],
"date-time": "2024-03-30T01:15:15Z",
"timestamp": 1711761315325
},
"is-referenced-by-count": 0,
"issue": "1",
"issued": {
"date-parts": [
[
2024,
3,
29
]
]
},
"journal-issue": {
"issue": "1",
"published-online": {
"date-parts": [
[
2024,
12
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2024,
3,
29
]
],
"date-time": "2024-03-29T00:00:00Z",
"timestamp": 1711670400000
}
},
{
"URL": "https://creativecommons.org/licenses/by/4.0",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2024,
3,
29
]
],
"date-time": "2024-03-29T00:00:00Z",
"timestamp": 1711670400000
}
}
],
"link": [
{
"URL": "https://www.nature.com/articles/s41598-024-57633-7.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://www.nature.com/articles/s41598-024-57633-7",
"content-type": "text/html",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://www.nature.com/articles/s41598-024-57633-7.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "297",
"original-title": [],
"prefix": "10.1038",
"published": {
"date-parts": [
[
2024,
3,
29
]
]
},
"published-online": {
"date-parts": [
[
2024,
3,
29
]
]
},
"publisher": "Springer Science and Business Media LLC",
"reference": [
{
"DOI": "10.1056/NEJMoa2204919",
"author": "R Arbel",
"doi-asserted-by": "publisher",
"first-page": "790",
"issue": "9",
"journal-title": "N. Engl. J. Med.",
"key": "57633_CR1",
"unstructured": "Arbel, R. et al. Nirmatrelvir use and severe Covid-19 outcomes during the omicron surge. N. Engl. J. Med. 387(9), 790–798 (2022).",
"volume": "387",
"year": "2022"
},
{
"DOI": "10.7326/M22-2141",
"author": "S Dryden-Peterson",
"doi-asserted-by": "publisher",
"first-page": "77",
"issue": "1",
"journal-title": "Ann. Intern. Med.",
"key": "57633_CR2",
"unstructured": "Dryden-Peterson, S. et al. Nirmatrelvir plus ritonavir for early COVID-19 and hospitalization in a large US health system. Ann. Intern. Med. 176(1), 77–84 (2023).",
"volume": "176",
"year": "2023"
},
{
"DOI": "10.1056/NEJMoa2118542",
"author": "J Hammond",
"doi-asserted-by": "publisher",
"first-page": "1397",
"issue": "15",
"journal-title": "N. Engl. J. Med.",
"key": "57633_CR3",
"unstructured": "Hammond, J. et al. Oral nirmatrelvir for high-risk, nonhospitalized adults with Covid-19. N. Engl. J. Med. 386(15), 1397–1408 (2022).",
"volume": "386",
"year": "2022"
},
{
"DOI": "10.1016/S1473-3099(23)00118-4",
"author": "JA Lewnard",
"doi-asserted-by": "publisher",
"first-page": "806",
"issue": "7",
"journal-title": "Lancet Infect. Dis.",
"key": "57633_CR4",
"unstructured": "Lewnard, J. A. et al. Effectiveness of nirmatrelvir–ritonavir in preventing hospital admissions and deaths in people with COVID-19: A cohort study in a large US health-care system. Lancet Infect. Dis. 23(7), 806–815 (2023).",
"volume": "23",
"year": "2023"
},
{
"DOI": "10.1093/cid/ciac443",
"author": "R Najjar-Debbiny",
"doi-asserted-by": "publisher",
"first-page": "e342",
"issue": "3",
"journal-title": "Clin. Infect. Dis.",
"key": "57633_CR5",
"unstructured": "Najjar-Debbiny, R. et al. Effectiveness of paxlovid in reducing severe coronavirus disease 2019 and mortality in high-risk patients. Clin. Infect. Dis. 76(3), e342–e349 (2023).",
"volume": "76",
"year": "2023"
},
{
"DOI": "10.1016/S0140-6736(22)01586-0",
"author": "CKH Wong",
"doi-asserted-by": "publisher",
"first-page": "1213",
"issue": "10359",
"journal-title": "Lancet",
"key": "57633_CR6",
"unstructured": "Wong, C. K. H. et al. Real-world effectiveness of molnupiravir and nirmatrelvir plus ritonavir against mortality, hospitalisation, and in-hospital outcomes among community-dwelling, ambulatory patients with confirmed SARS-CoV-2 infection during the omicron wave in Hong Kong: An observational study. Lancet 400(10359), 1213–1222 (2022).",
"volume": "400",
"year": "2022"
},
{
"author": "NIH",
"key": "57633_CR7",
"unstructured": "NIH. Prioritization of Anti-SARS-CoV-2 Therapies for the Treatment of COVID-19 in Nonhospitalized Patients When There Are Logistical Constraints (National Institutes of Health, 2022).",
"volume-title": "Prioritization of Anti-SARS-CoV-2 Therapies for the Treatment of COVID-19 in Nonhospitalized Patients When There Are Logistical Constraints",
"year": "2022"
},
{
"DOI": "10.1001/jamanetworkopen.2021.34147",
"author": "S Magesh",
"doi-asserted-by": "publisher",
"issue": "11",
"journal-title": "JAMA Netw. Open",
"key": "57633_CR8",
"unstructured": "Magesh, S. et al. Disparities in COVID-19 outcomes by race, ethnicity, and socioeconomic status. JAMA Netw. Open 4(11), e2134147 (2021).",
"volume": "4",
"year": "2021"
},
{
"DOI": "10.15585/mmwr.mm7143a2",
"author": "TK Boehmer",
"doi-asserted-by": "publisher",
"first-page": "1359",
"journal-title": "MMWR Morb. Mortal. Wkly. Rep",
"key": "57633_CR9",
"unstructured": "Boehmer, T. K. et al. Racial and ethnic disparities in outpatient treatment of COVID-19—United States, January–July 2022. MMWR Morb. Mortal. Wkly. Rep 71, 1359–1365 (2022).",
"volume": "71",
"year": "2022"
},
{
"DOI": "10.1093/cid/ciac673",
"author": "S Ganatra",
"doi-asserted-by": "publisher",
"first-page": "563",
"issue": "4",
"journal-title": "Clin. Infect. Dis.",
"key": "57633_CR10",
"unstructured": "Ganatra, S. et al. Oral nirmatrelvir and ritonavir in non-hospitalized vaccinated patients with Covid-19. Clin. Infect. Dis. 76(4), 563–572 (2022).",
"volume": "76",
"year": "2022"
},
{
"DOI": "10.1093/cid/ciac687",
"author": "T Yip",
"doi-asserted-by": "publisher",
"first-page": "26",
"issue": "3",
"journal-title": "Clin. Infect. Dis.",
"key": "57633_CR11",
"unstructured": "Yip, T. et al. Impact of the use of oral antiviral agents on the risk of hospitalization in community COVID-19 patients. Clin. Infect. Dis. 76(3), 26-e33 (2022).",
"volume": "76",
"year": "2022"
},
{
"DOI": "10.7812/TPP/22.172",
"author": "A Davis",
"doi-asserted-by": "publisher",
"first-page": "87",
"issue": "2",
"journal-title": "Perm. J.",
"key": "57633_CR12",
"unstructured": "Davis, A. et al. Comparing Kaiser Permanente members to the general population: Implications for generalizability of research. Perm. J. 27(2), 87–92 (2023).",
"volume": "27",
"year": "2023"
},
{
"key": "57633_CR13",
"unstructured": "Underlying Medical Conditions Associated with Higher Risk for Severe COVID-19: Information for Healthcare Professionals. https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-care/underlyingconditions.html (2023)."
},
{
"key": "57633_CR14",
"unstructured": "Drug-Drug Interactions Between Ritonavir-Boosted Nirmatrelvir (Paxlovid) and Concomitant Medications. https://www.covid19treatmentguidelines.nih.gov/therapies/antivirals-including-antibody-products/ritonavir-boosted-nirmatrelvir--paxlovid-/paxlovid-drug-drug-interactions/ (2023)."
},
{
"key": "57633_CR15",
"unstructured": "FDA. PAXLOVID Patient Eligibility Screening Checklist Tool for Prescribers. February 19th 2024. https://www.fda.gov/media/158165/download (2022)."
},
{
"DOI": "10.1007/s11524-006-9094-x",
"author": "LC Messer",
"doi-asserted-by": "publisher",
"first-page": "1041",
"issue": "6",
"journal-title": "J. Urban Health",
"key": "57633_CR16",
"unstructured": "Messer, L. C. et al. The development of a standardized neighborhood deprivation index. J. Urban Health 83(6), 1041–1062 (2006).",
"volume": "83",
"year": "2006"
},
{
"DOI": "10.2196/41529",
"author": "DE Malden",
"doi-asserted-by": "publisher",
"issue": "12",
"journal-title": "JMIR Public Health Surveill.",
"key": "57633_CR17",
"unstructured": "Malden, D. E. et al. Natural language processing for improved characterization of COVID-19 symptoms: Observational study of 350,000 patients in a large integrated health care system. JMIR Public Health Surveill. 8(12), e41529 (2022).",
"volume": "8",
"year": "2022"
},
{
"author": "WP Glasheen",
"first-page": "188",
"issue": "4",
"journal-title": "Am. Health Drug Benefits",
"key": "57633_CR18",
"unstructured": "Glasheen, W. P. et al. Charlson comorbidity index: ICD-9 update and ICD-10 translation. Am. Health Drug Benefits 12(4), 188–197 (2019).",
"volume": "12",
"year": "2019"
},
{
"DOI": "10.1007/s40121-023-00910-1",
"author": "HJ Appaneal",
"doi-asserted-by": "publisher",
"first-page": "155",
"issue": "1",
"journal-title": "Infect. Dis. Ther.",
"key": "57633_CR19",
"unstructured": "Appaneal, H. J. et al. Nirmatrelvir/ritonavir utilization for the treatment of non-hospitalized adults with COVID-19 in the National Veterans Affairs (VA) Healthcare System. Infect. Dis. Ther. 13(1), 155–172 (2024).",
"volume": "13",
"year": "2024"
},
{
"DOI": "10.1016/S1473-3099(23)00011-7",
"author": "NR Aggarwal",
"doi-asserted-by": "publisher",
"first-page": "696",
"issue": "6",
"journal-title": "Lancet Infect. Dis.",
"key": "57633_CR20",
"unstructured": "Aggarwal, N. R. et al. Real-world use of nirmatrelvir–ritonavir in outpatients with COVID-19 during the era of omicron variants including BA.4 and BA.5 in Colorado, USA: A retrospective cohort study. Lancet Infect. Dis. 23(6), 696–705 (2023).",
"volume": "23",
"year": "2023"
},
{
"DOI": "10.1371/journal.pone.0276742",
"author": "C Jefferson",
"doi-asserted-by": "publisher",
"issue": "11",
"journal-title": "PLoS One",
"key": "57633_CR21",
"unstructured": "Jefferson, C. et al. Differences in COVID-19 testing and adverse outcomes by race, ethnicity, sex, and health system setting in a large diverse US cohort. PLoS One 17(11), e0276742 (2022).",
"volume": "17",
"year": "2022"
},
{
"DOI": "10.15585/mmwr.mm7125e1",
"author": "JA Gold",
"doi-asserted-by": "publisher",
"first-page": "825",
"journal-title": "MMWR Morb. Mortal. Wkly. Rep.",
"key": "57633_CR22",
"unstructured": "Gold, J. A. et al. Dispensing of oral antiviral drugs for treatment of COVID-19 by zip code-level social vulnerability—United States, December 23, 2021–May 21, 2022. MMWR Morb. Mortal. Wkly. Rep. 71, 825–829 (2022).",
"volume": "71",
"year": "2022"
},
{
"DOI": "10.15585/mmwr.mm7143a3",
"author": "M Sullivan",
"doi-asserted-by": "publisher",
"first-page": "1384",
"issue": "43",
"journal-title": "MMWR Morb. Mortal. Wkly. Rep.",
"key": "57633_CR23",
"unstructured": "Sullivan, M. et al. Notes from the field: Dispensing of oral antiviral drugs for treatment of COVID-19 by zip code-level social vulnerability—United States, December 23, 2021–August 28, 2022. MMWR Morb. Mortal. Wkly. Rep. 28(43), 1384–1385 (2022).",
"volume": "28",
"year": "2022"
},
{
"DOI": "10.1016/S0140-6736(20)30893-X",
"author": "AV Dorn",
"doi-asserted-by": "publisher",
"first-page": "1243",
"issue": "10232",
"journal-title": "Lancet",
"key": "57633_CR24",
"unstructured": "Dorn, A. V., Cooney, R. E. & Sabin, M. L. COVID-19 exacerbating inequalities in the US. Lancet 395(10232), 1243–1244 (2020).",
"volume": "395",
"year": "2020"
},
{
"DOI": "10.1093/cid/ciaa1729",
"author": "JM McLaughlin",
"doi-asserted-by": "publisher",
"first-page": "e1814",
"issue": "7",
"journal-title": "Clin. Infect. Dis.",
"key": "57633_CR25",
"unstructured": "McLaughlin, J. M. et al. County-level predictors of coronavirus disease 2019 (COVID-19) cases and deaths in the United States: What happened, and where do we go from here?. Clin. Infect. Dis. 73(7), e1814–e1821 (2021).",
"volume": "73",
"year": "2021"
},
{
"key": "57633_CR26",
"unstructured": "Disparities in the risk and outcomes of COVID-19. 19th February 2024. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/908434/Disparities_in_the_risk_and_outcomes_of_COVID_August_2020_update.pdf (2020)."
},
{
"DOI": "10.3390/v15020438",
"author": "T Manciulli",
"doi-asserted-by": "publisher",
"first-page": "438",
"issue": "2",
"journal-title": "Viruses",
"key": "57633_CR27",
"unstructured": "Manciulli, T. et al. Safety and efficacy of outpatient treatments for COVID-19: Real-life data from a regionwide cohort of high-risk patients in Tuscany, Italy (the FEDERATE Cohort). Viruses 15(2), 438 (2023).",
"volume": "15",
"year": "2023"
},
{
"key": "57633_CR28",
"unstructured": "Murphy, S., Samson, L. W. & Sommers, B. D. COVID-19 Antivirals Utilization: Geographic and Demographic Patterns of Treatment in 2022, 19th February 2024. https://aspe.hhs.gov/sites/default/files/documents/a19600d0dccfee0e9c595730d73fd66d/covid-antivirals-report.pdf (2022)."
},
{
"author": "C Betsch",
"first-page": "1255",
"issue": "10232",
"journal-title": "Lancet Public Health",
"key": "57633_CR29",
"unstructured": "Betsch, C., Wieler, L. H. & Habersaat, K. Monitoring behavioural insights related to COVID-19. Lancet Public Health 395(10232), 1255–1256 (2020).",
"volume": "395",
"year": "2020"
},
{
"DOI": "10.1016/j.socscimed.2020.113638",
"author": "T Callaghan",
"doi-asserted-by": "publisher",
"journal-title": "Soc. Sci. Med.",
"key": "57633_CR30",
"unstructured": "Callaghan, T. et al. Correlates and disparities of intention to vaccinate against COVID-19. Soc. Sci. Med. 272, 113638 (2021).",
"volume": "272",
"year": "2021"
},
{
"DOI": "10.1136/bmjopen-2020-048172",
"author": "MF Lindholt",
"doi-asserted-by": "publisher",
"issue": "6",
"journal-title": "BMJ Open",
"key": "57633_CR31",
"unstructured": "Lindholt, M. F., Jørgensen, F., Bor, A. & Petersen, M. B. Public acceptance of COVID-19 vaccines: Cross-national evidence on levels and individual-level predictors using observational data. BMJ Open 11(6), e048172 (2021).",
"volume": "11",
"year": "2021"
},
{
"DOI": "10.1080/14760584.2022.2104251",
"author": "CJ Floyd",
"doi-asserted-by": "publisher",
"first-page": "1487",
"issue": "10",
"journal-title": "Expert Rev. Vaccines",
"key": "57633_CR32",
"unstructured": "Floyd, C. J. et al. COVID-19 vaccination and mask wearing behaviors in the United States, August 2020–June 2021. Expert Rev. Vaccines 21(10), 1487–1493 (2022).",
"volume": "21",
"year": "2022"
},
{
"DOI": "10.1016/j.amjmed.2022.08.035",
"author": "PJ O'Hayer",
"doi-asserted-by": "publisher",
"first-page": "63",
"issue": "1",
"journal-title": "Am. J. Med.",
"key": "57633_CR33",
"unstructured": "O’Hayer, P. J. et al. Evolution of care and outcomes across surges in hospitalized patients with coronavirus disease 2019. Am. J. Med. 136(1), 63–71 (2023).",
"volume": "136",
"year": "2023"
},
{
"DOI": "10.1016/j.ajt.2022.12.004",
"author": "MM Shah",
"doi-asserted-by": "publisher",
"first-page": "150",
"issue": "1",
"journal-title": "Am. J. Transplant.",
"key": "57633_CR34",
"unstructured": "Shah, M. M. et al. Paxlovid associated with decreased hospitalization rate among adults with COVID-19—United States, April–September 2022. Am. J. Transplant. 23(1), 150–155 (2023).",
"volume": "23",
"year": "2023"
}
],
"reference-count": 34,
"references-count": 34,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.nature.com/articles/s41598-024-57633-7"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Multidisciplinary"
],
"subtitle": [],
"title": "Predictors of nirmatrelvir–ritonavir receipt among COVID-19 patients in a large US health system",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1007/springer_crossmark_policy",
"volume": "14"
}