Phase II study of bemnifosbuvir in high-risk participants in a hospital setting with moderate COVID-19
et al., Future Virology, doi:10.2217/fvl-2023-0064, NCT04396106, Jun 2023
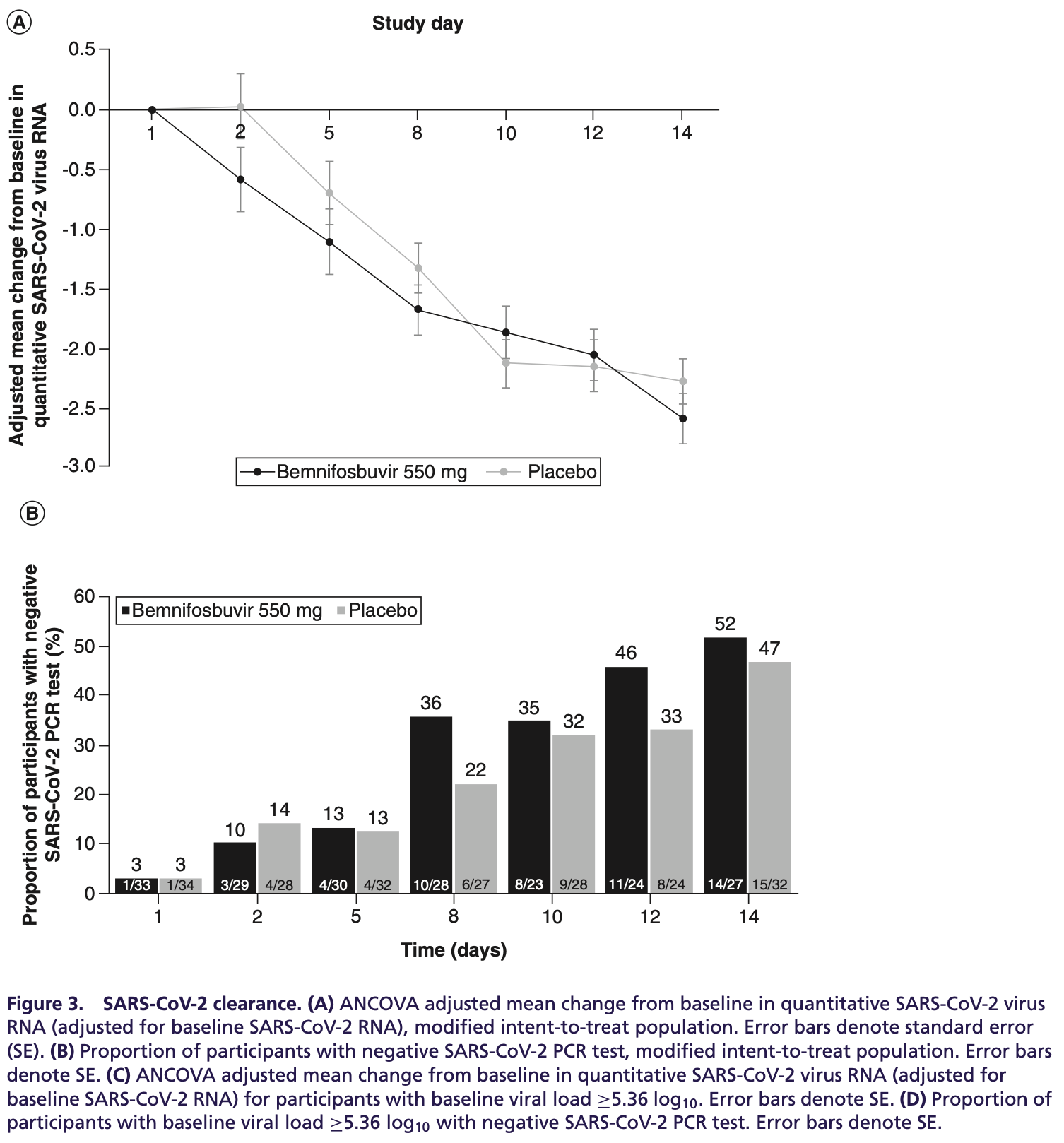
Phase 2 RCT investigating bemnifosbuvir for the treatment of 81 high-risk COVID-19 patients hospitalized with moderate disease. The trial was terminated early due to difficulties with enrollment. There was no significant difference between bemnifosbuvir and placebo for the primary outcome of disease progression or most secondary outcomes. However, viral load declined faster in the bemnifosbuvir group between days 2 and 8. All 3 deaths occurred in the placebo group. The safety profile was similar between groups. While results are very limited by small sample size and early termination, they suggest bemnifosbuvir may accelerate viral clearance and could play a role in preventing progression.
Viral load measured by PCR may not accurately reflect infectious virus measured by viral culture. Porter et al. show that viral load early in infection was correlated with infectious virus, but viral load late in infection could be high even with low or undetectable infectious virus. Assessing viral load later in infection may underestimate reductions in infectious virus with treatment.
Standard of Care (SOC) for COVID-19 in the study country,
the USA, is very poor with very low average efficacy for approved treatments2.
Only expensive, high-profit treatments were approved for early treatment. Low-cost treatments were excluded, reducing the probability of early treatment due to access and cost barriers, and eliminating complementary and synergistic benefits seen with many low-cost treatments.
|
risk of death, 85.6% lower, RR 0.14, p = 0.24, treatment 0 of 41 (0.0%), control 3 of 42 (7.1%), NNT 14, relative risk is not 0 because of continuity correction due to zero events (with reciprocal of the contrasting arm).
|
|
risk of progression, 26.8% lower, RR 0.73, p = 0.71, treatment 3 of 41 (7.3%), control 4 of 40 (10.0%), NNT 37, PRI.
|
|
risk of no recovery, 2.4% lower, RR 0.98, p = 1.00, treatment 8 of 41 (19.5%), control 8 of 40 (20.0%), NNT 205, clinical recovery.
|
|
risk of no viral clearance, 9.4% lower, RR 0.91, p = 0.80, treatment 13 of 27 (48.1%), control 17 of 32 (53.1%), NNT 20, day 14.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Horga et al., 23 Jun 2023, Double Blind Randomized Controlled Trial, placebo-controlled, USA, peer-reviewed, 8 authors, trial NCT04396106 (history).
Contact: horga.arantxa@ateapharma.com.
Phase II study of bemnifosbuvir in high-risk participants in a hospital setting with moderate COVID-19
Future Virology, doi:10.2217/fvl-2023-0064
Background: Bemnifosbuvir, a novel, oral, nonmutagenic, nonteratogenic nucleotide analogue inhibits SARS-CoV-2 replication in vitro. Materials & methods: Adults in hospital settings with moderate COVID-19 were randomized 1:1 bemnifosbuvir/placebo. Study amended to two parts after interim analysis; part B enrollment limited owing to evolving standard of care. Results: Although the study ended early and did not meet the primary efficacy end point, bemnifosbuvir was well tolerated and did not contribute to allcause mortality. Compared with placebo, bemnifosbuvir treatment resulted in 0.61 log 10 greater viral load mean change on day 2; trend sustained through day 8. Treatment-emergent adverse events were similar in both groups; most were mild/moderate, unrelated to study drug. Conclusion: Our results suggest a potential role for bemnifosbuvir in blunting COVID-19 progression. Clinical Trial Registration: NCT04396106 (ClinicalTrials.gov) Tweetable abstract: #Bemnifosbuvir, a novel/oral/nonmutagenic/nonteratogenic/nucleotide analogue with low DDI/resistance potential, inhibits SARS-CoV-2 replication and was well tolerated, did not contribute to all cause mortality and resulted in greater viral load mean change. Results suggest potential to blunt #COVID-19 progression.
Supplementary data To view the supplementary data that accompany this paper please visit the journal website at: www.futuremedicine.com/doi/ suppl/10.2217/fvl-2023-0064
Author contributions All authors were involved in the development of the study protocols; in the collection, analysis and interpretation of study data; in writing of the clinical study report; and in the decision to submit the article for publication.
Ethical conduct of research The authors state that they have obtained appropriate institutional review board approval and have followed the principles outlined in the Declaration of Helsinki for all human experimental investigations. In addition, informed consent has been obtained from the participants involved.
References
Clinicaltrials, Gov, SUNRISE-3: efficacy and safety of bemnifosbuvir in high-risk outpatients with COVID-19
Clinicaltrials, Gov, Safety and efficacy of AT-527 in subjects with moderate coronavirus disease (COVID-19) in a hospital setting
Fajnzylber, Regan, Coxen, SARS-CoV-2 viral load is associated with increased disease severity and mortality, Nat. Commun
Fusco, Shea, Lin, Health outcomes and economic burden of hospitalized COVID-19 patients in the United States, J. Med. Econ
Good, Westover, Jung, AT-527, a double prodrug of a guanosine nucleotide analog, is a potent inhibitor of SARS-CoV-2 in vitro and a promising oral antiviral for treatment of COVID-19, Antimicrob. Agents Chemother
Huang, Bemnifosbuvir, BEM, AT-527), a potent inhibitor of SARS-CoV-2 variants of concern (VOC), and a promising oral antiviral with a high resistance barrier for treatment of COVID-19 and other coronavirus infections
Islam, Hasan, Rahman, Islam, Comparative evaluation of authorized drugs for treating Covid-19 patients, Health Sci. Rep
Iuliano, Brunkard, Boehmer, Trends in disease severity and health care utilization during the early Omicron variant period compared with previous SARS-CoV-2 high transmission periods -United States, December 2020-January 2022, Morb. Mortal. Wkly. Rep
Jang, Choe, Yun, Reinfection with SARS-CoV-2 in general population, South Korea; nationwide retrospective cohort study, J. Med. Virol
Kip, Mccreary, Collins, Evolving real-world effectiveness of monoclonal antibodies for treatment of COVID-19: a cohort study, Ann. Intern. Med
Li, Hilgenfeld, Whitley, Clercq, Therapeutic strategies for COVID-19: progress and lessons learned, Nat. Rev. Drug Discov
Lin, Xu, Gu, Zeng, Sunny et al., Durability of bivalent boosters against omicron subvariants, N. Engl. J. Med
Marzolini, Kuritzkes, Marra, Recommendations for the management of drug-drug interactions between the COVID-19 antiviral nirmatrelvir/ritonavir (Paxlovid) and comedications, Clin. Pharmacol. Ther
Mazzitelli, Mengato, Sasset, Molnupiravir and nirmatrelvir/ritonavir: tolerability, safety, and adherence in a retrospective cohort study, Viruses
Paxlovid, Emergency Use Authorization Fact Sheet
Randall, Sam, Tartar, Murray, Cannon, More than 12.7 billion shots given: COVID-19 tracker
Salian, Wright, Vedell, COVID-19 transmission, current treatment, and future therapeutic strategies, Mol. Pharm
Sanderson, Hisner, Ia, Peacock, Ruis, Identification of a molnupiravir-associated mutational signature in SARS-CoV-2 sequencing databases, medRxiv, doi:10.1101/2023.01.26.23284998
Shannon, Fattorini, Sama, A dual mechanism of action of AT-527 against SARS-CoV-2 polymerase, Nat. Commun
Soriano, De-Mendoza, Edagwa, Oral antivirals for the prevention and treatment of SARS-CoV-2 infection, AIDS Rev
Vo, Good, Agrawal, Sommadossi, Low risk of drug-drug interactions (DDIs) for bemnifosbuvir (BEM) based upon in vitro metabolism and transporter interaction studies
Wang, Yang, Song, Oral GS-441524 derivatives: next-generation inhibitors of SARS-CoV-2 RNA-dependent RNA polymerase, Front. Immunol
Wong, Au, Lau, Lau, Cowling et al., Real-world effectiveness of early molnupiravir or nirmatrelvir-ritonavir in hospitalised patients with COVID-19 without supplemental oxygen requirement on admission during Hong Kong's omicron BA.2 wave: a retrospective cohort study, Lancet Infect. Dis
Zhou, Horga, Puri, AT-527 achieves antiviral concentrations in the human lung
Zhou, Morelli, Montrond, Bemnifosbuvir has low potential to interfere with P-gp, BCRP, and OATP1B1-mediated transport
Zhou, Morelli, Montround, No dose adjustments for CYP3A4 substrates when co-administered with bemnifosbuvir
DOI record:
{
"DOI": "10.2217/fvl-2023-0064",
"ISSN": [
"1746-0794",
"1746-0808"
],
"URL": "http://dx.doi.org/10.2217/fvl-2023-0064",
"abstract": "<jats:p> Background: Bemnifosbuvir, a novel, oral, nonmutagenic, nonteratogenic nucleotide analogue inhibits SARS-CoV-2 replication in vitro. Materials & methods: Adults in hospital settings with moderate COVID-19 were randomized 1:1 bemnifosbuvir/placebo. Study amended to two-parts after interim analysis; part B enrollment limited owing to evolving standard of care. Results: Although the study ended early and did not meet the primary efficacy end point, bemnifosbuvir was well tolerated and did not contribute to all-cause mortality. Compared with placebo, bemnifosbuvir treatment resulted in 0.61 log<jats:sub>10</jats:sub> greater viral load mean change on day 2; trend sustained through day 8. Treatment-emergent adverse events were similar in both groups; most were mild/moderate, unrelated to study drug. Conclusion: Our results suggest a potential role for bemnifosbuvir in blunting COVID-19 progression. </jats:p><jats:p> Clinical Trial Registration: NCT04396106 ( ClinicalTrials.gov ) </jats:p>",
"alternative-id": [
"10.2217/fvl-2023-0064"
],
"author": [
{
"affiliation": [
{
"name": "Atea Pharmaceuticals, Boston, MA 02110, USA"
}
],
"family": "Horga",
"given": "Arantxa",
"sequence": "first"
},
{
"affiliation": [
{
"name": "Brigham & Women's Hospital, Division of Infectious Disease, Boston, MA 02115, USA"
}
],
"family": "Kuritzkes",
"given": "Daniel R.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "American Institute of Research, Los Angeles, CA 90017, USA"
}
],
"family": "Kowalczyk",
"given": "John J.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Atea Pharmaceuticals, Boston, MA 02110, USA"
}
],
"family": "Pietropaolo",
"given": "Keith",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Atea Pharmaceuticals, Boston, MA 02110, USA"
}
],
"family": "Belanger",
"given": "Bruce",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Atea Pharmaceuticals, Boston, MA 02110, USA"
}
],
"family": "Lin",
"given": "Kai",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Atea Pharmaceuticals, Boston, MA 02110, USA"
}
],
"family": "Perkins",
"given": "Kristen",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Atea Pharmaceuticals, Boston, MA 02110, USA"
}
],
"family": "Hammond",
"given": "Janet",
"sequence": "additional"
}
],
"container-title": "Future Virology",
"container-title-short": "Future Virology",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2023,
6,
23
]
],
"date-time": "2023-06-23T11:05:27Z",
"timestamp": 1687518327000
},
"deposited": {
"date-parts": [
[
2023,
6,
23
]
],
"date-time": "2023-06-23T11:05:42Z",
"timestamp": 1687518342000
},
"funder": [
{
"name": "Atea Pharmaceuticals"
}
],
"indexed": {
"date-parts": [
[
2023,
6,
24
]
],
"date-time": "2023-06-24T04:27:40Z",
"timestamp": 1687580860503
},
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2023,
6,
23
]
]
},
"language": "en",
"link": [
{
"URL": "https://www.futuremedicine.com/doi/pdf/10.2217/fvl-2023-0064",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "1057",
"original-title": [],
"prefix": "10.2217",
"published": {
"date-parts": [
[
2023,
6,
23
]
]
},
"published-online": {
"date-parts": [
[
2023,
6,
23
]
]
},
"publisher": "Future Medicine Ltd",
"reference": [
{
"DOI": "10.1021/acs.molpharmaceut.0c00608",
"doi-asserted-by": "publisher",
"key": "B1"
},
{
"DOI": "10.1080/13696998.2021.1886109",
"doi-asserted-by": "publisher",
"key": "B2"
},
{
"DOI": "10.15585/mmwr.mm7104e4",
"doi-asserted-by": "publisher",
"key": "B3"
},
{
"author": "World Health Organization",
"key": "B4",
"volume-title": "Therapeutics and COVID-19: Living Guideline by World Health Organization.",
"year": "2021"
},
{
"key": "B5",
"unstructured": "FDA press release. FDA Approves First COVID-19 Vaccine. News Release. US FDA (2021). www.fda.gov/news-events/press-announcements/fda-approves-first-covid-19-vaccine"
},
{
"key": "B6",
"unstructured": "FDA press release. FDA Approves First Treatment For COVID-19. News release. US FDA (2020). www.fda.gov/news-events/press-announcements/fda-approves-first-treatment-covid-19#:∼:text=Today%2C%20the%20U.S.%20Food%20and,of%20COVID%2D19%20requiring%20hospitalization"
},
{
"key": "B7",
"unstructured": "Randall T, Sam C, Tartar A, Murray P, Cannon C. More than 12.7 billion shots given: COVID-19 tracker. Bloomberg (2022). www.bloomberg.com/graphics/covid-vaccine-tracker-global-distribution/"
},
{
"DOI": "10.1056/NEJMc2302462",
"doi-asserted-by": "publisher",
"key": "B8"
},
{
"DOI": "10.1002/jmv.28026",
"doi-asserted-by": "publisher",
"key": "B9"
},
{
"DOI": "10.7326/M22-1286",
"doi-asserted-by": "publisher",
"key": "B10"
},
{
"DOI": "10.1038/s41573-023-00672-y",
"doi-asserted-by": "publisher",
"key": "B11"
},
{
"author": "Wang Z",
"journal-title": "Front. Immunol.",
"key": "B12",
"volume": "13",
"year": "2022"
},
{
"key": "B13",
"unstructured": "CDC health advisory. COVID-19 rebound after paxlovid treatment. News release. Centers for Disease Control and Prevention (2022). https://emergency.cdc.gov/han/2022/pdf/CDC_HAN_467.pdf"
},
{
"DOI": "10.1002/cpt.2646",
"doi-asserted-by": "publisher",
"key": "B14"
},
{
"author": "Sanderson T",
"journal-title": "medRxiv",
"key": "B15",
"year": "2023"
},
{
"key": "B16",
"unstructured": "NIH treatment guidelines. Molnupiravir. NIH (2023). www.covid19treatmentguidelines.nih.gov/therapies/antivirals-including-antibody-products/molnupiravir/"
},
{
"key": "B17",
"unstructured": "Atea Pharmaceuticals press release. Atea Pharmaceuticals reports third quarter 2022 financial results and provides business update. News release. Atea Pharmaceuticals (2022). https://ir.ateapharma.com/news-releases/news-release-details/atea-pharmaceuticals-reports-third-quarter-2022-financial"
},
{
"DOI": "10.1002/hsr2.671",
"doi-asserted-by": "publisher",
"key": "B18"
},
{
"DOI": "10.24875/AIDSRev.22000001",
"doi-asserted-by": "publisher",
"key": "B19"
},
{
"DOI": "10.1038/s41467-022-28113-1",
"doi-asserted-by": "publisher",
"key": "B20"
},
{
"key": "B21",
"unstructured": "Atea Pharmaceuticals press release. Atea Pharmaceuticals reports first quarter 2023 financial results and provides business update. News release. Atea Pharmaceuticals (2023). https://ir.ateapharma.com/news-releases/news-release-details/atea-pharmaceuticals-reports-first-quarter-2023-financial"
},
{
"author": "Huang Q",
"key": "B22",
"volume-title": "International Conference on Antiviral Research.",
"year": "2023"
},
{
"author": "Vo A",
"key": "B23",
"volume-title": "International Conference on Antiviral Research.",
"year": "2023"
},
{
"author": "Zhou X-J",
"key": "B24",
"volume-title": "Conference on Retroviruses and Opportunistic Infections.",
"year": "2023"
},
{
"author": "Zhou XJ",
"key": "B25",
"volume-title": "Conference on Retroviruses and Opportunistic Infections.",
"year": "2023"
},
{
"author": "PAXLOVID",
"key": "B26",
"volume-title": "Emergency Use Authorization Fact Sheet.",
"year": "2023"
},
{
"key": "B27",
"unstructured": "Drug–drug interactions between ritonavir-boosted nirmatrelvir (paxlovid) and concomitant medications. NIH (2023). www.covid19treatmentguidelines.nih.gov/therapies/antivirals-including-antibody-products/ritonavir-boosted-nirmatrelvir-paxlovid-/paxlovid-drug-drug-interactions/"
},
{
"DOI": "10.1128/AAC.02479-20",
"doi-asserted-by": "publisher",
"key": "B28"
},
{
"author": "Zhou XJ",
"key": "B29",
"volume-title": "The 2021 ISIRV-WHO Virtual Conference.",
"year": "2021"
},
{
"key": "B30",
"unstructured": "Clinicaltrials.Gov. Safety and efficacy of AT-527 in subjects with moderate coronavirus disease (COVID-19) in a hospital setting (2020). https://clinicaltrials.gov/ct2/show/NCT04396106"
},
{
"key": "B31",
"unstructured": "FDA. Assessing COVID-19-related symptoms in outpatient adult and adolescent subjects in clinical trials of drugs and biological products for COVID-19 prevention or treatment. Guidance for industry. US Food and Drug Administration (2020). www.fda.gov/regulatory-information/search-fda-guidance-documents/assessing-covid-19-related-symptoms-outpatient-adult-and-adolescent-subjects-clinical-trials-drugs"
},
{
"DOI": "10.1038/s41467-020-19057-5",
"doi-asserted-by": "publisher",
"key": "B32"
},
{
"DOI": "10.1016/S1473-3099(22)00507-2",
"doi-asserted-by": "publisher",
"key": "B33"
},
{
"DOI": "10.3390/v15020384",
"doi-asserted-by": "publisher",
"key": "B34"
},
{
"key": "B35",
"unstructured": "Atea Pharmaceuticals press release. Atea Pharmaceuticals announces U.S. FDA fast track designation granted to bemnifosbuvir, an investigational oral antiviral, for the treatment of COVID-19. News release. Atea pharmaceuticals (2023). https://ir.ateapharma.com/news-releases/news-release-details/atea-pharmaceuticals-announces-us-fda-fast-track-designation-0"
},
{
"key": "B36",
"unstructured": "Atea Pharmaceuticals press release. Atea to advance global phase III registrational study of bemnifosbuvir in high-risk non-hospitalized patients with COVID-19. News release. Atea Pharmaceuticals (2022). https://ir.ateapharma.com/news-releases/news-release-details/atea-advance-global-phase-3-registrational-study-bemnifosbuvir"
},
{
"key": "B37",
"unstructured": "Clinicaltrials.Gov. SUNRISE-3: efficacy and safety of bemnifosbuvir in high-risk outpatients with COVID-19 (2022). https://clinicaltrials.gov/ct2/show/NCT05629962"
}
],
"reference-count": 37,
"references-count": 37,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.futuremedicine.com/doi/10.2217/fvl-2023-0064"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Virology"
],
"subtitle": [],
"title": "Phase II study of bemnifosbuvir in high-risk participants in a hospital setting with moderate COVID-19",
"type": "journal-article"
}