Comparative Pharmacovigilance Analysis of Approved and Repurposed Antivirals for COVID-19: Insights from EudraVigilance Data
et al., Biomedicines, doi:10.3390/biomedicines13061387, Jun 2025
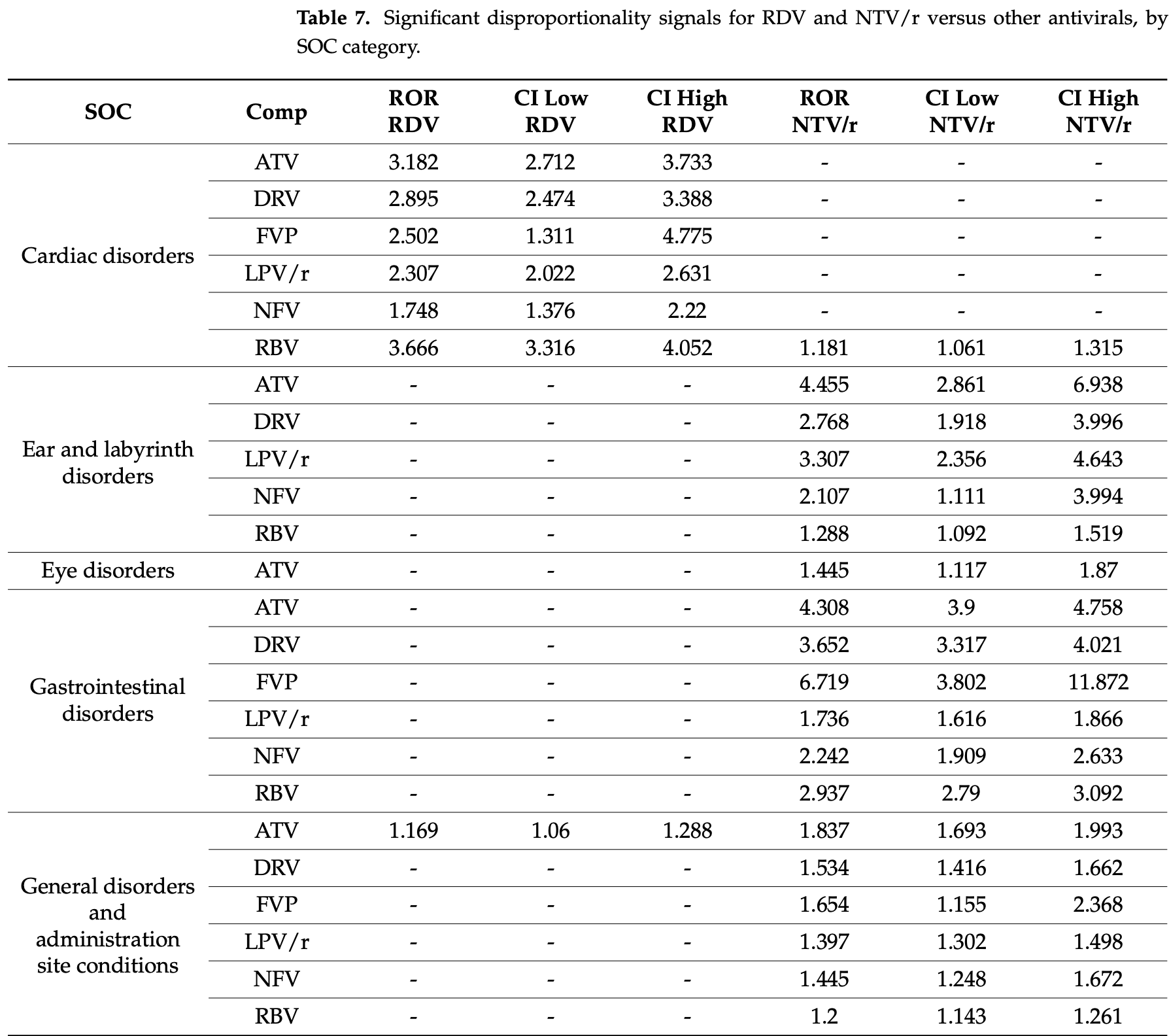
Pharmacovigilance analysis of 64,776 adverse event reports from EudraVigilance comparing safety profiles of COVID-19 antivirals, showing remdesivir associated with more serious adverse events compared to paxlovid. Remdesivir showed consistent safety signals for hepatobiliary, renal, cardiac, and general disorders, while paxlovid displayed signals for psychiatric, gastrointestinal disorders, and product issues.
Gérard, Zhou, Wu, Kamo, Choi, Kim show increased risk of acute kidney injury, Leo, Briciu, Muntean, Petrov show increased risk of liver injury, and Negru, Cheng, Mohammed, Kwok show increased risk of cardiac disorders with remdesivir.
Study covers remdesivir and paxlovid.
1.
Gérard et al., Remdesivir and Acute Renal Failure: A Potential Safety Signal From Disproportionality Analysis of the WHO Safety Database, Clinical Pharmacology & Therapeutics, doi:10.1002/cpt.2145.
2.
Zhou et al., Acute Kidney Injury and Drugs Prescribed for COVID-19 in Diabetes Patients: A Real-World Disproportionality Analysis, Frontiers in Pharmacology, doi:10.3389/fphar.2022.833679.
3.
Wu et al., Acute Kidney Injury Associated With Remdesivir: A Comprehensive Pharmacovigilance Analysis of COVID-19 Reports in FAERS, Frontiers in Pharmacology, doi:10.3389/fphar.2022.692828.
4.
Kamo et al., Association of Antiviral Drugs for the Treatment of COVID-19 With Acute Renal Failure, In Vivo, doi:10.21873/invivo.13637.
5.
Choi et al., Comparative effectiveness of combination therapy with nirmatrelvir–ritonavir and remdesivir versus monotherapy with remdesivir or nirmatrelvir–ritonavir in patients hospitalised with COVID-19: a target trial emulation study, The Lancet Infectious Diseases, doi:10.1016/S1473-3099(24)00353-0.
6.
Kim et al., Investigating the Safety Profile of Fast‐Track COVID‐19 Drugs Using the FDA Adverse Event Reporting System Database: A Comparative Observational Study, Pharmacoepidemiology and Drug Safety, doi:10.1002/pds.70043.
7.
Leo et al., Hepatocellular liver injury in hospitalized patients affected by COVID-19: Presence of different risk factors at different time points, Digestive and Liver Disease, doi:10.1016/j.dld.2021.12.014.
8.
Briciu et al., Evolving Clinical Manifestations and Outcomes in COVID-19 Patients: A Comparative Analysis of SARS-CoV-2 Variant Waves in a Romanian Hospital Setting, Pathogens, doi:10.3390/pathogens12121453.
9.
Muntean et al., Effects of COVID-19 on the Liver and Mortality in Patients with SARS-CoV-2 Pneumonia Caused by Delta and Non-Delta Variants: An Analysis in a Single Centre, Pharmaceuticals, doi:10.3390/ph17010003.
10.
Petrov et al., The Effect of Potentially Hepatotoxic Medicinal Products on Alanine Transaminase Levels in COVID-19 Patients: A Case–Control Study, Safety and Risk of Pharmacotherapy, doi:10.30895/2312-7821-2025-458.
11.
Negru et al., Comparative Pharmacovigilance Analysis of Approved and Repurposed Antivirals for COVID-19: Insights from EudraVigilance Data, Biomedicines, doi:10.3390/biomedicines13061387.
12.
Cheng et al., Cardiovascular Safety of COVID-19 Treatments: A Disproportionality Analysis of Adverse Event Reports from the WHO VigiBase, Infectious Diseases and Therapy, doi:10.1007/s40121-025-01225-z.
Negru et al., 5 Jun 2025, peer-reviewed, 6 authors.
Contact: dtit@uoradea.ro (corresponding author), negru.paulandrei@student.uoradea.ro, andreiflavius.radu@uoradea.ro, marin.ruxandracristina@student.uoradea.ro, raluca.aron@didactic.uoradea.ro, gbungau@uoradea.ro.
Comparative Pharmacovigilance Analysis of Approved and Repurposed Antivirals for COVID-19: Insights from EudraVigilance Data
Biomedicines, doi:10.3390/biomedicines13061387
Background/Objectives: During the COVID-19 pandemic, several antivirals were approved or repurposed, but their safety profiles have not been fully compared. Pharmacovigilance data help clarify how these drugs perform in real-world use. Methods: This study performed a comparative pharmacovigilance analysis of eight antivirals used or tested during the COVID-19 pandemic, based on individual case safety reports (ICSRs) retrieved from the EudraVigilance database, reported up to 9 February 2025 and extracted from the official platform on 12 February 2025. Adverse reactions were assessed by system organ class (SOC), demographic patterns, and seriousness, and disproportionality analysis (reporting odds ratio (ROR)) was conducted to identify potential safety signals. Results: A total of 64,776 ICSRs were analyzed. Among approved antivirals, nirmatrelvir/ritonavir (NTV/r) accounted for 13.4% (n = 8693) of reports, while remdesivir (RDV) represented 6.3% (n = 4105). Repurposed antivirals such as ribavirin and lopinavir/ritonavir dominated the dataset, together making up over 80% (n = 51,978) of all reports. RDV was associated with a high proportion of serious adverse events (84%, n = 3448), and showed consistent ROR signals in hepatobiliary, renal, cardiac, and general disorders, with values exceeding 2 in several comparisons. NTV/r displayed a milder overall profile, but with positive RORs for psychiatric disorders, gastrointestinal disorders, and product-related issues. The most affected SOCs across all drugs included general disorders (31.6%, n = 20,493), gastrointestinal (19.5%, n = 12,625), nervous system (17.8%, n = 11,511), and investigations (20.4%, n = 13,219). Demographic analysis showed that most events occurred in adults aged 18-64, with RDV more often reported in elderly patients and NTV/r more frequently associated with reports from female patients and non-healthcare reporters. Conclusions: This study highlights distinct pharmacovigilance profiles of COVID-19 antivirals and supports the role of real-world data in guiding safer therapeutic choices.
Funding: The APC is supported by the University of Oradea, Oradea, Romania. This research received no external funding from pharmaceutical companies. The authors and their institution (University of Oradea) have no financial ties to or sponsorship from manufacturers of the antivirals studied. Institutional Review Board Statement: Ethical review and approval were waived for this study due to the data used is anonymous, and patient identification is not possible through the information available in the EV database.
Conflicts of Interest: The authors declare no conflicts of interest.
Abbreviations The following abbreviations are used in this manuscript:
ADRs Adverse
References
Abedipour, Mirzaei, Ansari, Ehsanzadeh, Rashki et al., Remdesivir-Related Cardiac Adverse Effects in COVID-19 Patients: A Case-Control Study, Drug Res, doi:10.1055/a-2332-3253
Agrawal, Raju, Udwadia, Favipiravir: A New and Emerging Antiviral Option in COVID-19, Med. J. Armed Forces India, doi:10.1016/j.mjafi.2020.08.004
Aleem, Kothadia, Remdesivir, None
Alsowaida, Shehadeh, Kalligeros, Mylonakis, Incidence and Potential Risk Factors for Remdesivir-Associated Bradycardia in Hospitalized Patients with COVID-19: A Retrospective Cohort Study, Front. Pharmacol, doi:10.3389/fphar.2023.1106044
Atazanavir, None
Atazanavir, Summary of Product Characteristics
Bai, Du, Wang, Lau, Fung et al., Public Health Impact of Paxlovid as Treatment for COVID-19, United States, Emerg. Infect. Dis, doi:10.3201/eid3002.230835
Bate, Evans, Quantitative Signal Detection Using Spontaneous ADR Reporting, Pharmacoepidemiol. Drug Saf, doi:10.1002/pds.1742
Beigel, Tomashek, Dodd, Mehta, Zingman et al., Remdesivir for the Treatment of COVID-19-Final Report, N. Engl. J. Med, doi:10.1056/NEJMoa2007764
Bellino, COVID-19 Treatments Approved in the European Union and Clinical Recommendations for the Management of Non-Hospitalized and Hospitalized Patients, Ann. Med, doi:10.1080/07853890.2022.2133162
Bennett, Dolin, Blaser, Eds, Saunders, None
Blair, Remdesivir, A Review in COVID-19, Drugs, doi:10.1007/s40265-023-01926-0
Cascella, Rajnik, Cuomo, Dulebohn, Di Napoli et al., Evaluation, and Treatment of Coronavirus (COVID-19
Charan, Kaur, Bhardwaj, Haque, Sharma et al., Rapid Review of Suspected Adverse Drug Events Due to Remdesivir in the WHO Database; Findings and Implications, Expert Rev. Clin. Pharmacol, doi:10.1080/17512433.2021.1856655
Chouchana, Preta, Tisseyre, Terrier, Treluyer et al., Kidney Disorders as Serious Adverse Drug Reactions of Remdesivir in Coronavirus Disease 2019: A Retrospective Case-Noncase Study, Kidney Int, doi:10.1016/j.kint.2021.02.015
Choy, Wong, -L.; Kaewpreedee, Sia, Chen et al., Lopinavir, Emetine, and Homoharringtonine Inhibit SARS-CoV-2 Replication in Vitro, Antivir. Res, doi:10.1016/j.antiviral.2020.104786
Coste, Wong, Bokern, Bate, Douglas, Methods for Drug Safety Signal Detection Using Routinely Collected Observational Electronic Health Care Data: A Systematic Review, Pharmacoepidemiol. Drug Saf, doi:10.1002/pds.5548
Cozzupoli, Savastano, Falsini, Savastano, Rizzo, Possible Retinal Impairment Secondary to Ritonavir Use in SARS-CoV-2 Patients: A Narrative Systematic Review, J. Ophthalmol, doi:10.1155/2020/5350494
Darunavir, European Medicines Agency
De Meyer, Bojkova, Cinatl, Van Damme, Buyck et al., Lack of Antiviral Activity of Darunavir against SARS-CoV-2, Int. J. Infect. Dis, doi:10.1016/j.ijid.2020.05.085
Di Lenarda, Ferri, Lanzafame, Montuori, Pacelli, Cardiovascular Drug Interactions with Nirmatrelvir/Ritonavir for COVID-19: Considerations for Daily Practice, Eur. Cardiol, doi:10.15420/ecr.2024.04
Edwards, Aronson, Adverse Drug Reactions: Definitions, Diagnosis, and Management, Lancet, doi:10.1016/S0140-6736(00)02799-9
Eudravigilance, None
Fakhriravari, Malakouti, Remdesivir and the Liver: A Concise Narrative Review of Remdesivir-Associated Hepatotoxicity in Patients Hospitalized Due to COVID-19, Pharmacoepidemiology, doi:10.3390/pharma3010005
Fan, Zhang, Ma, Zhang, Safety Profile of the Antiviral Drug Remdesivir: An Update, Biomed. Pharmacother, doi:10.1016/j.biopha.2020.110532
Ferrara, Zovi, Trama, Vitiello, Nirmatrelvir-Remdesivir Association for Non-Hospitalized Adults with COVID-19, Point of View, Inflammopharmacology, doi:10.1007/s10787-022-01055-2
Ferreira-Da-Silva, Ribeiro-Vaz, Morato, Junqueira Polónia, A Comprehensive Review of Adverse Events to Drugs Used in COVID-19 Patients: Recent Clinical Evidence, Eur. J. Clin. Investig, doi:10.1111/eci.13763
Fintelman-Rodrigues, Sacramento, Ribeiro Lima, Souza Da Silva, Ferreira et al., Alone or in Combination with Ritonavir, Inhibits SARS-CoV-2 Replication and Proinflammatory Cytokine Production, Antimicrob. Agents Chemother, doi:10.1128/AAC.00825-20
Foo, Abdelnabi, Kaptein, Zhang, Ter Horst et al., HIV Protease Inhibitors Nelfinavir and Lopinavir/Ritonavir Markedly Improve Lung Pathology in SARS-CoV-2-Infected Syrian Hamsters despite Lack of an Antiviral Effect, Antivir. Res, doi:10.1016/j.antiviral.2022.105311
Fusaroli, Emanuel, Elisabetta, Hauben, The Evolving Role of Disproportionality Analysis in Pharmacovigilance, Expert Opin. Drug Saf, doi:10.1080/14740338.2024.2368817
Gandham, Eerike, Raj, Bisoi, Priyadarshini et al., Adverse Events Following Remdesivir Administration in Moderately Ill COVID-19 Patients-A Retrospective Analysis, J. Fam. Med. Prim. Care, doi:10.4103/jfmpc.jfmpc_2468_21
Ganipisetti, Bollimunta, Maringanti, Paxlovid-Induced Symptomatic Bradycardia and Syncope, Cureus, doi:10.7759/cureus.33831
Gao, Liu, Zou, Mao, Zhang, Effects of Nirmatrelvir/Ritonavir (Paxlovid) on the Nervous System: Analysis on Adverse Events Released by FDA, Expert Opin. Drug Saf, doi:10.1080/14740338.2025.2471509
Gidari, Sabbatini, Pallotto, Bastianelli, Pierucci et al., Nelfinavir: An Old Ally in the COVID-19 Fight?, Microorganisms, doi:10.3390/microorganisms10122471
Godwin, Polsonetti, Caron, Oppelt, Remdesivir for the Treatment of COVID-19: A Narrative Review, Infect. Dis. Ther, doi:10.1007/s40121-023-00900-3
Grein, Ohmagari, Shin, Diaz, Asperges et al., Compassionate Use of Remdesivir for Patients with Severe COVID-19, N. Engl. J. Med, doi:10.1056/NEJMoa2007016
Gu, Han, Wang, Zhang, The Impacts of Nirmatrelvir-Ritonavir on Myocardial Injury and Long-Term Cardiovascular Outcomes in Hospitalized Patients with COVID-19 amid the Omicron Wave of the Pandemic, Cardiovasc. Drugs Ther, doi:10.1007/s10557-024-07570-4
Gudima, Kofiadi, Shilovskiy, Kudlay, Khaitov, Antiviral Therapy of COVID-19, Int. J. Mol. Sci, doi:10.3390/ijms24108867
Gulick, Pau, Daar, Evans, Gandhi et al., National Institutes of Health COVID-19 Treatment Guidelines Panel: Perspectives and Lessons Learned, Ann. Intern. Med, doi:10.7326/ANNALS-24-00464
Han, Lee, Jung, Kim, Lee et al., Comparison of Agranulocytosis and Anti-Neutrophil Cytoplasmic Antibody-Associated Vasculitis Caused by Two Antithyroid Drugs: A Pharmacovigilance Study Using the WHO International Database, Fundam. Clin. Pharmacol, doi:10.1111/fcp.12991
Izcovich, Siemieniuk, Bartoszko, Ge, Zeraatkar et al., Adverse Effects of Remdesivir, Hydroxychloroquine and Lopinavir/Ritonavir When Used for COVID-19: Systematic Review and Meta-Analysis of Randomised Trials, BMJ Open, doi:10.1136/bmjopen-2020-048502
Kabir, Uddin, Hossain, Abdulhakim, Alam et al., NCOVID-19 Pandemic: From Molecular Pathogenesis to Potential Investigational Therapeutics, Front. Cell Dev. Biol, doi:10.3389/fcell.2020.00616
Kang, Kang, Im, Cho, Kang et al., Adverse Drug Events Associated with Remdesivir in Real-World Hospitalized Patients With COVID-19, Including Vulnerable Populations: A Retrospective Multicenter Study, J. Korean Med. Sci, doi:10.3346/jkms.2023.38.e346
Kim, Choi, Park, Kwon, Lee et al., Use of Darunavir-Cobicistat as a Treatment Option for Critically Ill Patients with SARS-CoV-2 Infection, Yonsei Med. J, doi:10.3349/ymj.2020.61.9.826
Lam, Patel, Nirmatrelvir-Ritonavir, None
Lamb, Nirmatrelvir Plus Ritonavir: First Approval, Drugs, doi:10.1007/s40265-022-01692-5
Li, Zhang, Liu, Wang, Liu, Adverse Events Associated with Nirmatrelvir/Ritonavir: A Pharmacovigilance Analysis Based on FAERS, Pharmaceuticals, doi:10.3390/ph15121455
Lopinavir, Highlights of Prescribing Information
Lopinavir, Ritonavir, Summary of Product Characteristics
Ma, Xie, Zhu, Yi, Zhao et al., Identification of Darunavir Derivatives for Inhibition of SARS-CoV-2 3CL(Pro), Int. J. Mol. Sci, doi:10.3390/ijms232416011
Montastruc, Thuriot, Durrieu, Hepatic Disorders With the Use of Remdesivir for Coronavirus 2019, Clin. Gastroenterol. Hepatol, doi:10.1016/j.cgh.2020.07.050
Nabati, Parsaee, Potential Cardiotoxic Effects of Remdesivir on Cardiovascular System: A Literature Review, Cardiovasc. Toxicol, doi:10.1007/s12012-021-09703-9
Negru, Radu, Vesa, Behl, Abdel-Daim et al., Therapeutic Dilemmas in Addressing SARS-CoV-2 Infection: Favipiravir versus Remdesivir, Biomed. Pharmacother, doi:10.1016/j.biopha.2022.112700
Nelfinavir, Summary of Product Characteristics
Nirmatrelvir, Ritonavir, Summary of Product Characteristics. Summary of Product Characteristics
Pacnejer, Negru, Arseniu, Trandafirescu, Oancea et al., Comparative Analysis of Neuropsychiatric Adverse Reactions Associated with Remdesivir and Nirmatrelvir/Ritonavir in COVID-19 Treatment: Insights from EudraVigilance Data, J. Clin. Med, doi:10.3390/jcm14061886
Park, Kim, Kim, Lee, Jung et al., Effectiveness and Adverse Events of Nirmatrelvir/Ritonavir Versus Molnupiravir for COVID-19 in Outpatient Setting: Multicenter Prospective Observational Study, J. Korean Med. Sci, doi:10.3346/jkms.2023.38.e347
Pop, Farcas, Butucă, Morgovan, Arseniu et al., Post-Marketing Surveillance of Statins-A Descriptive Analysis of Psychiatric Adverse Reactions in EudraVigilance, Pharmaceuticals, doi:10.3390/ph15121536
Pruijssers, George, Schäfer, Leist, Gralinksi et al., Remdesivir Inhibits SARS-CoV-2 in Human Lung Cells and Chimeric SARS-CoV Expressing the SARS-CoV-2 RNA Polymerase in Mice, Cell Rep, doi:10.1016/j.celrep.2020.107940
Rafaniello, Ferrajolo, Sullo, Gaio, Zinzi et al., Cardiac Events Potentially Associated to Remdesivir: An Analysis from the European Spontaneous Adverse Event Reporting System, Pharmaceuticals, doi:10.3390/ph14070611
Ray, Thomas, 156-Hepatitis, Mandell, Bennett's Principles and Practice of Infectious Diseases
Remdesivir, Summary of Product Characteristics
Ribavirin, European Medicines Agency
Ribavirin, Highlights of Prescribing Information
Ribavirin, Livertox, Clinical and Research Information on Drug-Induced Liver Injury
Romão, Duval, Lima, Da Silva, De Matos, Detection of Potential Safety Signals Related to the Use of Remdesivir and Tocilizumab in the COVID Era during Pregnancy, Resorting to Open Data from the FDA Adverse Event Reporting System (FAERS), Front. Pharmacol, doi:10.3389/fphar.2024.1349543
Salem, Manouchehri, Moey, Lebrun-Vignes, Bastarache et al., Cardiovascular Toxicities Associated with Immune Checkpoint Inhibitors: An Observational, Retrospective, Pharmacovigilance Study, Lancet Oncol, doi:10.1016/S1470-2045(18)30608-9
Santos, Rousseau, Gauthier, Calmy, Schneider, Patient Experiences With Nirmatrelvir/Ritonavir for COVID-19 in a Collaborative Care Model: A Cross-Sectional Study on Self-Management, Information, and Medication Impact, J. Patient Exp, doi:10.1177/23743735251342126
Schulz, Huynh, Heger, Bakir, Adverse Effects of Remdesivir for the Treatment of Acute COVID-19 in the Pediatric Population: A Retrospective Observational Study, Mol. Cell. Pediatr, doi:10.1186/s40348-024-00175-9
Silva, Zara, Figueras, De Melo, Potential Kidney Damage Associated with the Use of Remdesivir for COVID-19: Analysis of a Pharmacovigilance Database, Cad. Saude Publica, doi:10.1590/0102-311x00077721
Singh, De Wit, Antiviral Agents for the Treatment of COVID-19: Progress and Challenges, Cell Rep. Med, doi:10.1016/j.xcrm.2022.100549
Sullivan, Two by Two Tables Containing Counts (TwobyTwo). OpenEpi: Open Source Epidemiologic Statistics for Public Health
Sun, Deng, Huang, He, Huang, Data Mining of Adverse Drug Event Signals with Nirmatrelvir/Ritonavir from FAERS, PLoS ONE, doi:10.1371/journal.pone.0316573
Veklury, European Medicines Agency
Vonica, Butuca, Morgovan, Pumnea, Cipaian et al., Bevacizumab-Insights from EudraVigilance Database on the Assessments of the Safety Profile of Monoclonal Antibodies Used as Targeted Cancer Treatment, Pharmaceuticals, doi:10.3390/ph18040501
Watson, Barnsley, Toor, Hogan, Winskill et al., Global Impact of the First Year of COVID-19 Vaccination: A Mathematical Modelling Study, Lancet. Infect. Dis, doi:10.1016/S1473-3099(22)00320-6
Wee, Lim, Tay, Chiew, Young et al., Nirmatrelvir/Ritonavir Treatment and Risk for Postacute Sequelae of COVID-19 in Older Singaporeans, Clin. Microbiol. Infect, doi:10.1016/j.cmi.2024.08.019
Yadav, Chaudhary, Jain, Chaudhary, Khanra et al., Role of Structural and Non-Structural Proteins and Therapeutic Targets of SARS-CoV-2 for COVID-19, Cells, doi:10.3390/cells10040821
Yang, Jung, Kim, Lee, Yon et al., Cardiovascular and Cerebrovascular Adverse Events Associated with Intravitreal Anti-VEGF Monoclonal Antibodies: A World Health Organization Pharmacovigilance Study, Ophthalmology, doi:10.1016/j.ophtha.2024.07.008
Yang, Wei, Chang, Chang, Tsai et al., Remdesivir Use in the Coronavirus Disease 2019 Pandemic: A Mini-Review, J. Microbiol. Immunol. Infect, doi:10.1016/j.jmii.2020.09.002
Zorych, Madigan, Ryan, Bate, Disproportionality Methods for Pharmacovigilance in Longitudinal Observational Databases, Stat. Methods Med. Res, doi:10.1177/0962280211403602
DOI record:
{
"DOI": "10.3390/biomedicines13061387",
"ISSN": [
"2227-9059"
],
"URL": "http://dx.doi.org/10.3390/biomedicines13061387",
"abstract": "<jats:p>Background/Objectives: During the COVID-19 pandemic, several antivirals were approved or repurposed, but their safety profiles have not been fully compared. Pharmacovigilance data help clarify how these drugs perform in real-world use. Methods: This study performed a comparative pharmacovigilance analysis of eight antivirals used or tested during the COVID-19 pandemic, based on individual case safety reports (ICSRs) retrieved from the EudraVigilance database, reported up to 9 February 2025 and extracted from the official platform on 12 February 2025. Adverse reactions were assessed by system organ class (SOC), demographic patterns, and seriousness, and disproportionality analysis (reporting odds ratio (ROR)) was conducted to identify potential safety signals. Results: A total of 64,776 ICSRs were analyzed. Among approved antivirals, nirmatrelvir/ritonavir (NTV/r) accounted for 13.4% (n = 8693) of reports, while remdesivir (RDV) represented 6.3% (n = 4105). Repurposed antivirals such as ribavirin and lopinavir/ritonavir dominated the dataset, together making up over 80% (n = 51,978) of all reports. RDV was associated with a high proportion of serious adverse events (84%, n = 3448), and showed consistent ROR signals in hepatobiliary, renal, cardiac, and general disorders, with values exceeding 2 in several comparisons. NTV/r displayed a milder overall profile, but with positive RORs for psychiatric disorders, gastrointestinal disorders, and product-related issues. The most affected SOCs across all drugs included general disorders (31.6%, n = 20,493), gastrointestinal (19.5%, n = 12,625), nervous system (17.8%, n = 11,511), and investigations (20.4%, n = 13,219). Demographic analysis showed that most events occurred in adults aged 18–64, with RDV more often reported in elderly patients and NTV/r more frequently associated with reports from female patients and non-healthcare reporters. Conclusions: This study highlights distinct pharmacovigilance profiles of COVID-19 antivirals and supports the role of real-world data in guiding safer therapeutic choices.</jats:p>",
"alternative-id": [
"biomedicines13061387"
],
"author": [
{
"affiliation": [
{
"name": "Doctoral School of Biological and Biomedical Sciences, University of Oradea, 410087 Oradea, Romania"
},
{
"name": "Department of Preclinical Disciplines, Faculty of Medicine and Pharmacy, University of Oradea, 410073 Oradea, Romania"
}
],
"family": "Negru",
"given": "Paul Andrei",
"sequence": "first"
},
{
"ORCID": "https://orcid.org/0000-0002-0296-6592",
"affiliation": [
{
"name": "Doctoral School of Biological and Biomedical Sciences, University of Oradea, 410087 Oradea, Romania"
},
{
"name": "Department of Pharmacy, Faculty of Medicine and Pharmacy, University of Oradea, 410028 Oradea, Romania"
}
],
"authenticated-orcid": false,
"family": "Tit",
"given": "Delia Mirela",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0002-7625-8177",
"affiliation": [
{
"name": "Doctoral School of Biological and Biomedical Sciences, University of Oradea, 410087 Oradea, Romania"
},
{
"name": "Department of Psycho-Neurosciences and Recovery, Faculty of Medicine and Pharmacy, University of Oradea, 410073 Oradea, Romania"
}
],
"authenticated-orcid": false,
"family": "Radu",
"given": "Andrei Flavius",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Doctoral School of Biological and Biomedical Sciences, University of Oradea, 410087 Oradea, Romania"
}
],
"family": "Bungau",
"given": "Gabriela",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0002-4425-215X",
"affiliation": [
{
"name": "Department of Preclinical Disciplines, Faculty of Medicine and Pharmacy, University of Oradea, 410073 Oradea, Romania"
}
],
"authenticated-orcid": false,
"family": "Corb Aron",
"given": "Raluca Anca",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0002-3182-1683",
"affiliation": [
{
"name": "Doctoral School of Biological and Biomedical Sciences, University of Oradea, 410087 Oradea, Romania"
}
],
"authenticated-orcid": false,
"family": "Marin",
"given": "Ruxandra Cristina",
"sequence": "additional"
}
],
"container-title": "Biomedicines",
"container-title-short": "Biomedicines",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2025,
6,
5
]
],
"date-time": "2025-06-05T12:34:32Z",
"timestamp": 1749126872000
},
"deposited": {
"date-parts": [
[
2025,
6,
6
]
],
"date-time": "2025-06-06T10:49:52Z",
"timestamp": 1749206992000
},
"funder": [
{
"name": "University of Oradea, Oradea, Romania"
}
],
"indexed": {
"date-parts": [
[
2025,
6,
6
]
],
"date-time": "2025-06-06T11:10:05Z",
"timestamp": 1749208205658,
"version": "3.41.0"
},
"is-referenced-by-count": 0,
"issue": "6",
"issued": {
"date-parts": [
[
2025,
6,
5
]
]
},
"journal-issue": {
"issue": "6",
"published-online": {
"date-parts": [
[
2025,
6
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2025,
6,
5
]
],
"date-time": "2025-06-05T00:00:00Z",
"timestamp": 1749081600000
}
}
],
"link": [
{
"URL": "https://www.mdpi.com/2227-9059/13/6/1387/pdf",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "1968",
"original-title": [],
"page": "1387",
"prefix": "10.3390",
"published": {
"date-parts": [
[
2025,
6,
5
]
]
},
"published-online": {
"date-parts": [
[
2025,
6,
5
]
]
},
"publisher": "MDPI AG",
"reference": [
{
"key": "ref_1",
"unstructured": "Cascella, M., Rajnik, M., Cuomo, A., Dulebohn, S.C., and Di Napoli, R. (2025, April 14). Features, Evaluation, and Treatment of Coronavirus (COVID-19), Available online: https://www.ncbi.nlm.nih.gov/books/NBK554776/."
},
{
"key": "ref_2",
"unstructured": "(2025, April 17). COVID-19 Cases, World. World Health Organization COVID-19 Dashboard. Available online: https://data.who.int/dashboards/covid19/cases?n=c."
},
{
"DOI": "10.1016/S1473-3099(22)00320-6",
"article-title": "Global Impact of the First Year of COVID-19 Vaccination: A Mathematical Modelling Study",
"author": "Watson",
"doi-asserted-by": "crossref",
"first-page": "1293",
"journal-title": "Lancet. Infect. Dis.",
"key": "ref_3",
"volume": "22",
"year": "2022"
},
{
"DOI": "10.3390/ijms24108867",
"doi-asserted-by": "crossref",
"key": "ref_4",
"unstructured": "Gudima, G., Kofiadi, I., Shilovskiy, I., Kudlay, D., and Khaitov, M. (2023). Antiviral Therapy of COVID-19. Int. J. Mol. Sci., 24."
},
{
"DOI": "10.7326/ANNALS-24-00464",
"article-title": "National Institutes of Health COVID-19 Treatment Guidelines Panel: Perspectives and Lessons Learned",
"author": "Gulick",
"doi-asserted-by": "crossref",
"first-page": "1547",
"journal-title": "Ann. Intern. Med.",
"key": "ref_5",
"volume": "177",
"year": "2024"
},
{
"key": "ref_6",
"unstructured": "(2025, April 15). Coronavirus Disease 2019 (COVID-19) Treatment Guidelines. National Institutes of Health, Available online: https://www.ncbi.nlm.nih.gov/books/NBK570371/pdf/Bookshelf_NBK570371.pdf."
},
{
"DOI": "10.1016/j.biopha.2022.112700",
"doi-asserted-by": "crossref",
"key": "ref_7",
"unstructured": "Negru, P.A., Radu, A.-F., Vesa, C.M., Behl, T., Abdel-Daim, M.M., Nechifor, A.C., Endres, L., Stoicescu, M., Pasca, B., and Tit, D.M. (2022). Therapeutic Dilemmas in Addressing SARS-CoV-2 Infection: Favipiravir versus Remdesivir. Biomed. Pharmacother., 147."
},
{
"DOI": "10.3390/ijms232416011",
"doi-asserted-by": "crossref",
"key": "ref_8",
"unstructured": "Ma, L., Xie, Y., Zhu, M., Yi, D., Zhao, J., Guo, S., Zhang, Y., Wang, J., Li, Q., and Wang, Y. (2022). Identification of Darunavir Derivatives for Inhibition of SARS-CoV-2 3CL(Pro). Int. J. Mol. Sci., 23."
},
{
"DOI": "10.1016/j.antiviral.2020.104786",
"article-title": "Remdesivir, Lopinavir, Emetine, and Homoharringtonine Inhibit SARS-CoV-2 Replication in Vitro",
"author": "Choy",
"doi-asserted-by": "crossref",
"first-page": "104786",
"journal-title": "Antivir. Res.",
"key": "ref_9",
"volume": "178",
"year": "2020"
},
{
"DOI": "10.3389/fcell.2020.00616",
"doi-asserted-by": "crossref",
"key": "ref_10",
"unstructured": "Kabir, M.T., Uddin, M.S., Hossain, M.F., Abdulhakim, J.A., Alam, M.A., Ashraf, G.M., Bungau, S.G., Bin-Jumah, M.N., Abdel-Daim, M.M., and Aleya, L. (2020). NCOVID-19 Pandemic: From Molecular Pathogenesis to Potential Investigational Therapeutics. Front. Cell Dev. Biol., 8."
},
{
"DOI": "10.1016/j.celrep.2020.107940",
"article-title": "Remdesivir Inhibits SARS-CoV-2 in Human Lung Cells and Chimeric SARS-CoV Expressing the SARS-CoV-2 RNA Polymerase in Mice",
"author": "Pruijssers",
"doi-asserted-by": "crossref",
"first-page": "107940",
"journal-title": "Cell Rep.",
"key": "ref_11",
"volume": "32",
"year": "2020"
},
{
"DOI": "10.1007/s40265-023-01926-0",
"article-title": "Remdesivir: A Review in COVID-19",
"author": "Blair",
"doi-asserted-by": "crossref",
"first-page": "1215",
"journal-title": "Drugs",
"key": "ref_12",
"volume": "83",
"year": "2023"
},
{
"DOI": "10.1007/s40121-023-00900-3",
"article-title": "Remdesivir for the Treatment of COVID-19: A Narrative Review",
"author": "Godwin",
"doi-asserted-by": "crossref",
"first-page": "1",
"journal-title": "Infect. Dis. Ther.",
"key": "ref_13",
"volume": "13",
"year": "2024"
},
{
"DOI": "10.1007/s12012-021-09703-9",
"article-title": "Potential Cardiotoxic Effects of Remdesivir on Cardiovascular System: A Literature Review",
"author": "Nabati",
"doi-asserted-by": "crossref",
"first-page": "268",
"journal-title": "Cardiovasc. Toxicol.",
"key": "ref_14",
"volume": "22",
"year": "2022"
},
{
"DOI": "10.1016/j.ophtha.2024.07.008",
"article-title": "Cardiovascular and Cerebrovascular Adverse Events Associated with Intravitreal Anti-VEGF Monoclonal Antibodies: A World Health Organization Pharmacovigilance Study",
"author": "Yang",
"doi-asserted-by": "crossref",
"first-page": "62",
"journal-title": "Ophthalmology",
"key": "ref_15",
"volume": "132",
"year": "2025"
},
{
"DOI": "10.1007/s10787-022-01055-2",
"article-title": "Nirmatrelvir-Remdesivir Association for Non-Hospitalized Adults with COVID-19, Point of View",
"author": "Ferrara",
"doi-asserted-by": "crossref",
"first-page": "1927",
"journal-title": "Inflammopharmacology",
"key": "ref_16",
"volume": "30",
"year": "2022"
},
{
"DOI": "10.3201/eid3002.230835",
"article-title": "Public Health Impact of Paxlovid as Treatment for COVID-19, United States",
"author": "Bai",
"doi-asserted-by": "crossref",
"first-page": "262",
"journal-title": "Emerg. Infect. Dis.",
"key": "ref_17",
"volume": "30",
"year": "2024"
},
{
"key": "ref_18",
"unstructured": "Aleem, A., and Kothadia, J.P. (2025, May 25). Remdesivir, Available online: https://www.ncbi.nlm.nih.gov/books/NBK563261/."
},
{
"DOI": "10.1080/07853890.2022.2133162",
"article-title": "COVID-19 Treatments Approved in the European Union and Clinical Recommendations for the Management of Non-Hospitalized and Hospitalized Patients",
"author": "Bellino",
"doi-asserted-by": "crossref",
"first-page": "2856",
"journal-title": "Ann. Med.",
"key": "ref_19",
"volume": "54",
"year": "2022"
},
{
"key": "ref_20",
"unstructured": "(2025, May 25). Veklury. European Medicines Agency. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/veklury."
},
{
"key": "ref_21",
"unstructured": "(2025, May 25). Introduction to Therapeutics and COVID-19. World Health Organization. Available online: https://www.who.int/docs/default-source/coronaviruse/module-1-introduction-to-therapeutics-for-covid-19.pdf."
},
{
"DOI": "10.1007/s40265-022-01692-5",
"article-title": "Nirmatrelvir Plus Ritonavir: First Approval",
"author": "Lamb",
"doi-asserted-by": "crossref",
"first-page": "585",
"journal-title": "Drugs",
"key": "ref_22",
"volume": "82",
"year": "2022"
},
{
"key": "ref_23",
"unstructured": "(2025, May 25). Paxlovid. European Medicines Agency. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/paxlovid."
},
{
"key": "ref_24",
"unstructured": "(2025, May 26). Coronavirus (COVID-19) Update: FDA Authorizes First Oral Antiviral for Treatment of COVID-19, Available online: https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-first-oral-antiviral-treatment-covid-19."
},
{
"DOI": "10.1016/j.mjafi.2020.08.004",
"article-title": "Favipiravir: A New and Emerging Antiviral Option in COVID-19",
"author": "Agrawal",
"doi-asserted-by": "crossref",
"first-page": "370",
"journal-title": "Med. J. Armed Forces India",
"key": "ref_25",
"volume": "76",
"year": "2020"
},
{
"key": "ref_26",
"unstructured": "(2025, May 25). Report on the Deliberation Results. Review Report. Favipiravir, Available online: https://www.pmda.go.jp/files/000210319.pdf."
},
{
"key": "ref_27",
"unstructured": "(2025, May 25). Lopinavir/Ritonavir. Summary of Product Characteristics. Available online: https://www.ema.europa.eu/en/documents/product-information/lopinavir-ritonavir-viatris-epar-product-information_en.pdf."
},
{
"key": "ref_28",
"unstructured": "(2025, May 25). Lopinavir/Ritonavir. Rezumatul Caracteristicilor Produsului. Available online: https://ec.europa.eu/health/documents/community-register/2016/20160114133755/anx_133755_ro.pdf."
},
{
"key": "ref_29",
"unstructured": "Lopinavir and Ritonavir (2025, May 25). Highlights of Prescribing Information, Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/021251s052_021906s046lbl.pdf."
},
{
"key": "ref_30",
"unstructured": "Bennett, J.E., Dolin, R., and Blaser, M.J. (2015). 156–Hepatitis C. Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases, W.B. Saunders. [8th ed.]."
},
{
"key": "ref_31",
"unstructured": "(2025, May 25). Ribavirin. Highlights of Prescribing Information, Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2013/020903s052,021546s008lbl.pdf."
},
{
"key": "ref_32",
"unstructured": "(2025, May 25). Ribavirin. European Medicines Agency. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/ribavirin-teva."
},
{
"key": "ref_33",
"unstructured": "(2025, May 25). Nelfinavir. Summary of Product Characteristics. Available online: https://ec.europa.eu/health/documents/community-register/2010/2010012073128/anx_73128_en.pdf."
},
{
"DOI": "10.3390/microorganisms10122471",
"doi-asserted-by": "crossref",
"key": "ref_34",
"unstructured": "Gidari, A., Sabbatini, S., Pallotto, C., Bastianelli, S., Pierucci, S., Busti, C., Schiaroli, E., and Francisci, D. (2022). Nelfinavir: An Old Ally in the COVID-19 Fight?. Microorganisms, 10."
},
{
"DOI": "10.1016/j.antiviral.2022.105311",
"article-title": "HIV Protease Inhibitors Nelfinavir and Lopinavir/Ritonavir Markedly Improve Lung Pathology in SARS-CoV-2-Infected Syrian Hamsters despite Lack of an Antiviral Effect",
"author": "Foo",
"doi-asserted-by": "crossref",
"first-page": "105311",
"journal-title": "Antivir. Res.",
"key": "ref_35",
"volume": "202",
"year": "2022"
},
{
"key": "ref_36",
"unstructured": "(2025, May 25). Atazanavir. Summary of Product Characteristics. Available online: https://www.ema.europa.eu/en/documents/product-information/atazanavir-krka-epar-product-information_en.pdf."
},
{
"key": "ref_37",
"unstructured": "(2025, May 25). Atazanavir, Available online: https://clinicalinfo.hiv.gov/en/drugs/atazanavir/patient."
},
{
"article-title": "Atazanavir, Alone or in Combination with Ritonavir, Inhibits SARS-CoV-2 Replication and Proinflammatory Cytokine Production",
"author": "Sacramento",
"first-page": "1",
"journal-title": "Antimicrob. Agents Chemother.",
"key": "ref_38",
"volume": "64",
"year": "2020"
},
{
"key": "ref_39",
"unstructured": "(2025, May 25). Darunavir. European Medicines Agency. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/darunavir-krka."
},
{
"DOI": "10.1016/j.ijid.2020.05.085",
"article-title": "Lack of Antiviral Activity of Darunavir against SARS-CoV-2",
"author": "Bojkova",
"doi-asserted-by": "crossref",
"first-page": "7",
"journal-title": "Int. J. Infect. Dis.",
"key": "ref_40",
"volume": "97",
"year": "2020"
},
{
"DOI": "10.3349/ymj.2020.61.9.826",
"article-title": "Use of Darunavir-Cobicistat as a Treatment Option for Critically Ill Patients with SARS-CoV-2 Infection",
"author": "Kim",
"doi-asserted-by": "crossref",
"first-page": "826",
"journal-title": "Yonsei Med. J.",
"key": "ref_41",
"volume": "61",
"year": "2020"
},
{
"key": "ref_42",
"unstructured": "(2025, May 24). EudraVigilance. European Medicines Agency. Available online: https://www.ema.europa.eu/en/human-regulatory-overview/research-development/pharmacovigilance-research-development/eudravigilance."
},
{
"key": "ref_43",
"unstructured": "European Medicines Agency (2025, February 12). Online Access to Suspected Side-Effect Reports: EudraVigilance—European Database of Suspected Adverse Drug Reaction Reports. Available online: https://www.adrreports.eu."
},
{
"key": "ref_44",
"unstructured": "(2025, February 26). Introductory Guide for Standardised MedDRA Queries (SMQs) Version 27.0. The International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use. Available online: https://admin.meddra.org/sites/default/files/guidance/file/SMQ_intguide_27_0_English.pdf."
},
{
"key": "ref_45",
"unstructured": "International Council for Harmonisation (ICH) (2025, February 26). Clinical Safety Data Management: Definitions and Standards for Expedited Reporting (E2A). Available online: https://database.ich.org/sites/default/files/E2A_Guideline.pdf."
},
{
"key": "ref_46",
"unstructured": "(2025, April 12). Open Source Epidemiologic Statistics for Public Health. Available online: https://www.openepi.com/Menu/OE_Menu.htm."
},
{
"DOI": "10.3390/ph18040501",
"doi-asserted-by": "crossref",
"key": "ref_47",
"unstructured": "Vonica, R.C., Butuca, A., Morgovan, C., Pumnea, M., Cipaian, R.C., Frum, A., Dobrea, C.M., Vonica-Tincu, A.L., Pacnejer, A.-M., and Ghibu, S. (2025). Bevacizumab—Insights from EudraVigilance Database on the Assessments of the Safety Profile of Monoclonal Antibodies Used as Targeted Cancer Treatment. Pharmaceuticals, 18."
},
{
"DOI": "10.3390/ph15121536",
"doi-asserted-by": "crossref",
"key": "ref_48",
"unstructured": "Pop, G., Farcaș, A., Butucă, A., Morgovan, C., Arseniu, A.M., Pumnea, M., Teodoru, M., and Gligor, F.G. (2022). Post-Marketing Surveillance of Statins-A Descriptive Analysis of Psychiatric Adverse Reactions in EudraVigilance. Pharmaceuticals, 15."
},
{
"key": "ref_49",
"unstructured": "Sullivan, K. (2025, February 26). Two by Two Tables Containing Counts (TwobyTwo). OpenEpi: Open Source Epidemiologic Statistics for Public Health. Available online: https://www.openepi.com/PDFDocs/TwobyTwoDoc.pdf."
},
{
"key": "ref_50",
"unstructured": "(2025, April 12). Screening for Adverse Drug Reactions in EudraVigilance. European Medicines Agency. Available online: https://policycommons.net/artifacts/3181767/screening-for-adverse-reactions-in-eudravigilance/3980283/."
},
{
"DOI": "10.1080/14740338.2024.2368817",
"article-title": "The Evolving Role of Disproportionality Analysis in Pharmacovigilance",
"author": "Fusaroli",
"doi-asserted-by": "crossref",
"first-page": "981",
"journal-title": "Expert Opin. Drug Saf.",
"key": "ref_51",
"volume": "23",
"year": "2024"
},
{
"DOI": "10.1016/j.xcrm.2022.100549",
"article-title": "Antiviral Agents for the Treatment of COVID-19: Progress and Challenges",
"author": "Singh",
"doi-asserted-by": "crossref",
"first-page": "100549",
"journal-title": "Cell Rep. Med.",
"key": "ref_52",
"volume": "3",
"year": "2022"
},
{
"DOI": "10.3346/jkms.2023.38.e346",
"article-title": "Adverse Drug Events Associated with Remdesivir in Real-World Hospitalized Patients With COVID-19, Including Vulnerable Populations: A Retrospective Multicenter Study",
"author": "Kang",
"doi-asserted-by": "crossref",
"first-page": "e346",
"journal-title": "J. Korean Med. Sci.",
"key": "ref_53",
"volume": "38",
"year": "2023"
},
{
"DOI": "10.3346/jkms.2023.38.e347",
"article-title": "Effectiveness and Adverse Events of Nirmatrelvir/Ritonavir Versus Molnupiravir for COVID-19 in Outpatient Setting: Multicenter Prospective Observational Study",
"author": "Park",
"doi-asserted-by": "crossref",
"first-page": "e347",
"journal-title": "J. Korean Med. Sci.",
"key": "ref_54",
"volume": "38",
"year": "2023"
},
{
"key": "ref_55",
"unstructured": "(2025, May 26). Remdesivir. Summary of Product Characteristics. Available online: https://www.ema.europa.eu/en/documents/product-information/veklury-epar-product-information_en.pdf."
},
{
"DOI": "10.3390/ph14070611",
"doi-asserted-by": "crossref",
"key": "ref_56",
"unstructured": "Rafaniello, C., Ferrajolo, C., Sullo, M.G., Gaio, M., Zinzi, A., Scavone, C., Gargano, F., Coscioni, E., Rossi, F., and Capuano, A. (2021). Cardiac Events Potentially Associated to Remdesivir: An Analysis from the European Spontaneous Adverse Event Reporting System. Pharmaceuticals, 14."
},
{
"DOI": "10.1186/s40348-024-00175-9",
"article-title": "Adverse Effects of Remdesivir for the Treatment of Acute COVID-19 in the Pediatric Population: A Retrospective Observational Study",
"author": "Schulz",
"doi-asserted-by": "crossref",
"first-page": "2",
"journal-title": "Mol. Cell. Pediatr.",
"key": "ref_57",
"volume": "11",
"year": "2024"
},
{
"DOI": "10.3389/fphar.2023.1106044",
"doi-asserted-by": "crossref",
"key": "ref_58",
"unstructured": "Alsowaida, Y.S., Shehadeh, F., Kalligeros, M., and Mylonakis, E. (2023). Incidence and Potential Risk Factors for Remdesivir-Associated Bradycardia in Hospitalized Patients with COVID-19: A Retrospective Cohort Study. Front. Pharmacol., 14."
},
{
"DOI": "10.1055/a-2332-3253",
"article-title": "Remdesivir-Related Cardiac Adverse Effects in COVID-19 Patients: A Case-Control Study",
"author": "Abedipour",
"doi-asserted-by": "crossref",
"first-page": "290",
"journal-title": "Drug Res.",
"key": "ref_59",
"volume": "74",
"year": "2024"
},
{
"DOI": "10.1016/j.cgh.2020.07.050",
"article-title": "Hepatic Disorders With the Use of Remdesivir for Coronavirus 2019",
"author": "Montastruc",
"doi-asserted-by": "crossref",
"first-page": "2835",
"journal-title": "Clin. Gastroenterol. Hepatol.",
"key": "ref_60",
"volume": "18",
"year": "2020"
},
{
"DOI": "10.3390/pharma3010005",
"article-title": "Remdesivir and the Liver: A Concise Narrative Review of Remdesivir-Associated Hepatotoxicity in Patients Hospitalized Due to COVID-19",
"author": "FakhriRavari",
"doi-asserted-by": "crossref",
"first-page": "69",
"journal-title": "Pharmacoepidemiology",
"key": "ref_61",
"volume": "3",
"year": "2024"
},
{
"DOI": "10.1056/NEJMoa2007016",
"article-title": "Compassionate Use of Remdesivir for Patients with Severe COVID-19",
"author": "Grein",
"doi-asserted-by": "crossref",
"first-page": "2327",
"journal-title": "N. Engl. J. Med.",
"key": "ref_62",
"volume": "382",
"year": "2020"
},
{
"DOI": "10.3390/cells10040821",
"doi-asserted-by": "crossref",
"key": "ref_63",
"unstructured": "Yadav, R., Chaudhary, J.K., Jain, N., Chaudhary, P.K., Khanra, S., Dhamija, P., Sharma, A., Kumar, A., and Handu, S. (2021). Role of Structural and Non-Structural Proteins and Therapeutic Targets of SARS-CoV-2 for COVID-19. Cells, 10."
},
{
"DOI": "10.3389/fphar.2024.1349543",
"doi-asserted-by": "crossref",
"key": "ref_64",
"unstructured": "Romão, B.M.S., Duval, F.V., Lima, E.C., da Silva, F.A.B., and de Matos, G.C. (2024). Detection of Potential Safety Signals Related to the Use of Remdesivir and Tocilizumab in the COVID Era during Pregnancy, Resorting to Open Data from the FDA Adverse Event Reporting System (FAERS). Front. Pharmacol., 15."
},
{
"DOI": "10.1016/j.biopha.2020.110532",
"doi-asserted-by": "crossref",
"key": "ref_65",
"unstructured": "Fan, Q., Zhang, B., Ma, J., and Zhang, S. (2020). Safety Profile of the Antiviral Drug Remdesivir: An Update. Biomed. Pharmacother., 130."
},
{
"DOI": "10.4103/jfmpc.jfmpc_2468_21",
"article-title": "Adverse Events Following Remdesivir Administration in Moderately Ill COVID-19 Patients—A Retrospective Analysis",
"author": "Gandham",
"doi-asserted-by": "crossref",
"first-page": "3693",
"journal-title": "J. Fam. Med. Prim. Care",
"key": "ref_66",
"volume": "11",
"year": "2022"
},
{
"DOI": "10.1111/eci.13763",
"article-title": "A Comprehensive Review of Adverse Events to Drugs Used in COVID-19 Patients: Recent Clinical Evidence",
"author": "Morato",
"doi-asserted-by": "crossref",
"first-page": "e13763",
"journal-title": "Eur. J. Clin. Investig.",
"key": "ref_67",
"volume": "52",
"year": "2022"
},
{
"DOI": "10.1016/j.jmii.2020.09.002",
"article-title": "Remdesivir Use in the Coronavirus Disease 2019 Pandemic: A Mini-Review",
"author": "Yang",
"doi-asserted-by": "crossref",
"first-page": "27",
"journal-title": "J. Microbiol. Immunol. Infect.",
"key": "ref_68",
"volume": "54",
"year": "2021"
},
{
"DOI": "10.1590/0102-311x00077721",
"article-title": "Potential Kidney Damage Associated with the Use of Remdesivir for COVID-19: Analysis of a Pharmacovigilance Database",
"author": "Silva",
"doi-asserted-by": "crossref",
"first-page": "e00077721",
"journal-title": "Cad. Saude Publica",
"key": "ref_69",
"volume": "37",
"year": "2021"
},
{
"DOI": "10.1016/j.kint.2021.02.015",
"article-title": "Kidney Disorders as Serious Adverse Drug Reactions of Remdesivir in Coronavirus Disease 2019: A Retrospective Case-Noncase Study",
"author": "Chouchana",
"doi-asserted-by": "crossref",
"first-page": "1235",
"journal-title": "Kidney Int.",
"key": "ref_70",
"volume": "99",
"year": "2021"
},
{
"DOI": "10.1136/bmjopen-2020-048502",
"article-title": "Adverse Effects of Remdesivir, Hydroxychloroquine and Lopinavir/Ritonavir When Used for COVID-19: Systematic Review and Meta-Analysis of Randomised Trials",
"author": "Izcovich",
"doi-asserted-by": "crossref",
"first-page": "e048502",
"journal-title": "BMJ Open",
"key": "ref_71",
"volume": "12",
"year": "2022"
},
{
"DOI": "10.1080/17512433.2021.1856655",
"article-title": "Rapid Review of Suspected Adverse Drug Events Due to Remdesivir in the WHO Database; Findings and Implications",
"author": "Charan",
"doi-asserted-by": "crossref",
"first-page": "95",
"journal-title": "Expert Rev. Clin. Pharmacol.",
"key": "ref_72",
"volume": "14",
"year": "2020"
},
{
"DOI": "10.1056/NEJMoa2007764",
"article-title": "Remdesivir for the Treatment of COVID-19—Final Report",
"author": "Beigel",
"doi-asserted-by": "crossref",
"first-page": "1813",
"journal-title": "N. Engl. J. Med.",
"key": "ref_73",
"volume": "383",
"year": "2020"
},
{
"key": "ref_74",
"unstructured": "(2025, May 25). Nirmatrelvir/Ritonavir. Summary of Product Characteristics. Summary of Product Characteristics. European Medicines Agency. Available online: https://www.ema.europa.eu/en/documents/product-information/paxlovid-epar-product-information_en.pdf."
},
{
"DOI": "10.1007/s10557-024-07570-4",
"article-title": "The Impacts of Nirmatrelvir-Ritonavir on Myocardial Injury and Long-Term Cardiovascular Outcomes in Hospitalized Patients with COVID-19 amid the Omicron Wave of the Pandemic",
"author": "Gu",
"doi-asserted-by": "crossref",
"first-page": "573",
"journal-title": "Cardiovasc. Drugs Ther.",
"key": "ref_75",
"volume": "39",
"year": "2025"
},
{
"DOI": "10.15420/ecr.2024.04",
"article-title": "Cardiovascular Drug Interactions with Nirmatrelvir/Ritonavir for COVID-19: Considerations for Daily Practice",
"author": "Ferri",
"doi-asserted-by": "crossref",
"first-page": "e15",
"journal-title": "Eur. Cardiol.",
"key": "ref_76",
"volume": "19",
"year": "2024"
},
{
"DOI": "10.1016/j.cmi.2024.08.019",
"article-title": "Nirmatrelvir/Ritonavir Treatment and Risk for Postacute Sequelae of COVID-19 in Older Singaporeans",
"author": "Wee",
"doi-asserted-by": "crossref",
"first-page": "93",
"journal-title": "Clin. Microbiol. Infect.",
"key": "ref_77",
"volume": "31",
"year": "2025"
},
{
"article-title": "Paxlovid-Induced Symptomatic Bradycardia and Syncope",
"author": "Ganipisetti",
"first-page": "e33831",
"journal-title": "Cureus",
"key": "ref_78",
"volume": "15",
"year": "2023"
},
{
"DOI": "10.1155/2020/5350494",
"article-title": "Possible Retinal Impairment Secondary to Ritonavir Use in SARS-CoV-2 Patients: A Narrative Systematic Review",
"author": "Cozzupoli",
"doi-asserted-by": "crossref",
"first-page": "5350494",
"journal-title": "J. Ophthalmol.",
"key": "ref_79",
"volume": "2020",
"year": "2020"
},
{
"DOI": "10.3390/ph15121455",
"doi-asserted-by": "crossref",
"key": "ref_80",
"unstructured": "Li, M., Zhang, Q.-S., Liu, X.-L., Wang, H.-L., and Liu, W. (2022). Adverse Events Associated with Nirmatrelvir/Ritonavir: A Pharmacovigilance Analysis Based on FAERS. Pharmaceuticals, 15."
},
{
"DOI": "10.1177/23743735251342126",
"article-title": "Patient Experiences With Nirmatrelvir/Ritonavir for COVID-19 in a Collaborative Care Model: A Cross-Sectional Study on Self-Management, Information, and Medication Impact",
"author": "Santos",
"doi-asserted-by": "crossref",
"first-page": "23743735251342126",
"journal-title": "J. Patient Exp.",
"key": "ref_81",
"volume": "12",
"year": "2025"
},
{
"DOI": "10.1371/journal.pone.0316573",
"doi-asserted-by": "crossref",
"key": "ref_82",
"unstructured": "Sun, J., Deng, X., Huang, J., He, G., and Huang, S. (2024). Data Mining of Adverse Drug Event Signals with Nirmatrelvir/Ritonavir from FAERS. PLoS ONE, 19."
},
{
"DOI": "10.1080/14740338.2025.2471509",
"doi-asserted-by": "crossref",
"key": "ref_83",
"unstructured": "Gao, C., Liu, Z., Zou, Z., Mao, L., and Zhang, J. (2025). Effects of Nirmatrelvir/Ritonavir (Paxlovid) on the Nervous System: Analysis on Adverse Events Released by FDA. Expert Opin. Drug Saf., 1–8."
},
{
"DOI": "10.3390/jcm14061886",
"doi-asserted-by": "crossref",
"key": "ref_84",
"unstructured": "Pacnejer, A.-M., Negru, M.C., Arseniu, A.M., Trandafirescu, C., Oancea, C., Gligor, F.G., Morgovan, C., Butuca, A., and Dehelean, C.A. (2025). Comparative Analysis of Neuropsychiatric Adverse Reactions Associated with Remdesivir and Nirmatrelvir/Ritonavir in COVID-19 Treatment: Insights from EudraVigilance Data. J. Clin. Med., 14."
},
{
"key": "ref_85",
"unstructured": "Lam, C., and Patel, P. (2025, May 26). Nirmatrelvir-Ritonavir, Available online: https://www.ncbi.nlm.nih.gov/books/NBK585126/."
},
{
"key": "ref_86",
"unstructured": "(2025, April 14). Ribavirin. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury, Available online: https://www.ncbi.nlm.nih.gov/books/NBK548115/."
},
{
"DOI": "10.1177/0962280211403602",
"article-title": "Disproportionality Methods for Pharmacovigilance in Longitudinal Observational Databases",
"author": "Zorych",
"doi-asserted-by": "crossref",
"first-page": "39",
"journal-title": "Stat. Methods Med. Res.",
"key": "ref_87",
"volume": "22",
"year": "2013"
},
{
"DOI": "10.1002/pds.1742",
"article-title": "Quantitative Signal Detection Using Spontaneous ADR Reporting",
"author": "Bate",
"doi-asserted-by": "crossref",
"first-page": "427",
"journal-title": "Pharmacoepidemiol. Drug Saf.",
"key": "ref_88",
"volume": "18",
"year": "2009"
},
{
"DOI": "10.1002/pds.5548",
"article-title": "Methods for Drug Safety Signal Detection Using Routinely Collected Observational Electronic Health Care Data: A Systematic Review",
"author": "Coste",
"doi-asserted-by": "crossref",
"first-page": "28",
"journal-title": "Pharmacoepidemiol. Drug Saf.",
"key": "ref_89",
"volume": "32",
"year": "2023"
},
{
"DOI": "10.1016/S0140-6736(00)02799-9",
"article-title": "Adverse Drug Reactions: Definitions, Diagnosis, and Management",
"author": "Edwards",
"doi-asserted-by": "crossref",
"first-page": "1255",
"journal-title": "Lancet",
"key": "ref_90",
"volume": "356",
"year": "2000"
},
{
"DOI": "10.1111/fcp.12991",
"article-title": "Comparison of Agranulocytosis and Anti-Neutrophil Cytoplasmic Antibody-Associated Vasculitis Caused by Two Antithyroid Drugs: A Pharmacovigilance Study Using the WHO International Database",
"author": "Han",
"doi-asserted-by": "crossref",
"first-page": "780",
"journal-title": "Fundam. Clin. Pharmacol.",
"key": "ref_91",
"volume": "38",
"year": "2024"
},
{
"DOI": "10.1016/S1470-2045(18)30608-9",
"article-title": "Cardiovascular Toxicities Associated with Immune Checkpoint Inhibitors: An Observational, Retrospective, Pharmacovigilance Study",
"author": "Salem",
"doi-asserted-by": "crossref",
"first-page": "1579",
"journal-title": "Lancet Oncol.",
"key": "ref_92",
"volume": "19",
"year": "2018"
}
],
"reference-count": 92,
"references-count": 92,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.mdpi.com/2227-9059/13/6/1387"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Comparative Pharmacovigilance Analysis of Approved and Repurposed Antivirals for COVID-19: Insights from EudraVigilance Data",
"type": "journal-article",
"volume": "13"
}