Cardiovascular Safety of COVID-19 Treatments: A Disproportionality Analysis of Adverse Event Reports from the WHO VigiBase
et al., Infectious Diseases and Therapy, doi:10.1007/s40121-025-01225-z, Sep 2025
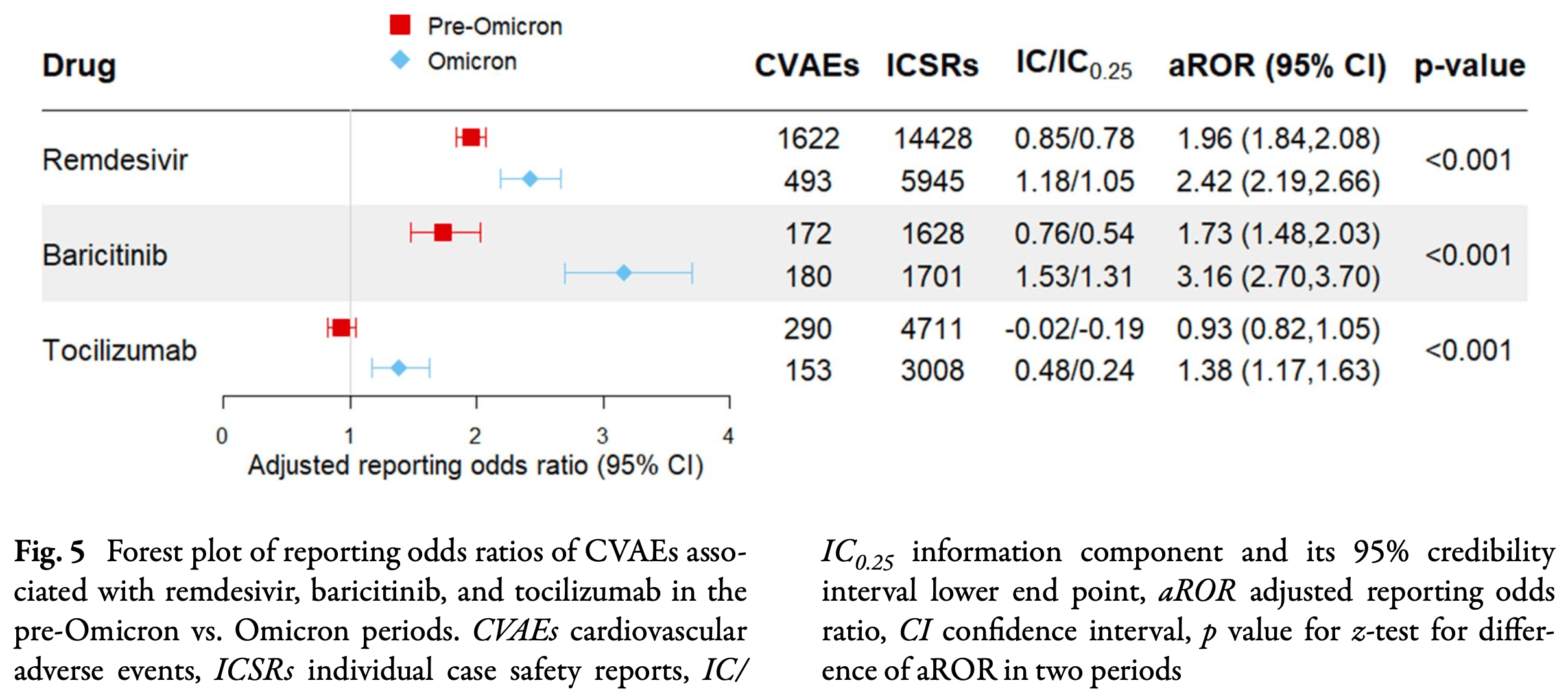
Retrospective disproportionality analysis of 276,631 COVID-19 adverse event reports from the WHO VigiBase database showing that remdesivir and baricitinib were associated with 2.4-fold and 2.3-fold increased odds of cardiovascular adverse events, and that cardiovascular adverse events were unexpectedly higher for Omicron compared to pre-Omicron variants for remdesivir, baricitinib, and tocilizumab.
Gérard, Zhou, Wu, Kamo, Choi, Kim show increased risk of acute kidney injury, Leo, Briciu, Muntean, Petrov show increased risk of liver injury, and Negru, Cheng, Mohammed, Kwok show increased risk of cardiac disorders with remdesivir.
Study covers remdesivir and tocilizumab.
1.
Gérard et al., Remdesivir and Acute Renal Failure: A Potential Safety Signal From Disproportionality Analysis of the WHO Safety Database, Clinical Pharmacology & Therapeutics, doi:10.1002/cpt.2145.
2.
Zhou et al., Acute Kidney Injury and Drugs Prescribed for COVID-19 in Diabetes Patients: A Real-World Disproportionality Analysis, Frontiers in Pharmacology, doi:10.3389/fphar.2022.833679.
3.
Wu et al., Acute Kidney Injury Associated With Remdesivir: A Comprehensive Pharmacovigilance Analysis of COVID-19 Reports in FAERS, Frontiers in Pharmacology, doi:10.3389/fphar.2022.692828.
4.
Kamo et al., Association of Antiviral Drugs for the Treatment of COVID-19 With Acute Renal Failure, In Vivo, doi:10.21873/invivo.13637.
5.
Choi et al., Comparative effectiveness of combination therapy with nirmatrelvir–ritonavir and remdesivir versus monotherapy with remdesivir or nirmatrelvir–ritonavir in patients hospitalised with COVID-19: a target trial emulation study, The Lancet Infectious Diseases, doi:10.1016/S1473-3099(24)00353-0.
6.
Kim et al., Investigating the Safety Profile of Fast‐Track COVID‐19 Drugs Using the FDA Adverse Event Reporting System Database: A Comparative Observational Study, Pharmacoepidemiology and Drug Safety, doi:10.1002/pds.70043.
7.
Leo et al., Hepatocellular liver injury in hospitalized patients affected by COVID-19: Presence of different risk factors at different time points, Digestive and Liver Disease, doi:10.1016/j.dld.2021.12.014.
8.
Briciu et al., Evolving Clinical Manifestations and Outcomes in COVID-19 Patients: A Comparative Analysis of SARS-CoV-2 Variant Waves in a Romanian Hospital Setting, Pathogens, doi:10.3390/pathogens12121453.
9.
Muntean et al., Effects of COVID-19 on the Liver and Mortality in Patients with SARS-CoV-2 Pneumonia Caused by Delta and Non-Delta Variants: An Analysis in a Single Centre, Pharmaceuticals, doi:10.3390/ph17010003.
10.
Petrov et al., The Effect of Potentially Hepatotoxic Medicinal Products on Alanine Transaminase Levels in COVID-19 Patients: A Case–Control Study, Safety and Risk of Pharmacotherapy, doi:10.30895/2312-7821-2025-458.
11.
Negru et al., Comparative Pharmacovigilance Analysis of Approved and Repurposed Antivirals for COVID-19: Insights from EudraVigilance Data, Biomedicines, doi:10.3390/biomedicines13061387.
12.
Cheng et al., Cardiovascular Safety of COVID-19 Treatments: A Disproportionality Analysis of Adverse Event Reports from the WHO VigiBase, Infectious Diseases and Therapy, doi:10.1007/s40121-025-01225-z.
Cheng et al., 11 Sep 2025, retrospective, peer-reviewed, 5 authors, study period March 2020 - July 2023.
Contact: bryan.yan@cuhk.edu.hk, ellen.poon@cuhk.edu.hk.
Cardiovascular Safety of COVID-19 Treatments: A Disproportionality Analysis of Adverse Event Reports from the WHO VigiBase
Infectious Diseases and Therapy, doi:10.1007/s40121-025-01225-z
Introduction: The high mortality of Coronavirus Disease 2019 (COVID-19) highlights the need for safe and effective antiviral treatment. Small molecular antivirals (remdesivir, molnupiravir, nirmatrelvir/ritonavir) and immunomodulators (baricitinib, tocilizumab) have been developed or repurposed to suppress viral replication and ameliorate cytokine storms, respectively. Despite U.S. Food and Drug Administration (FDA) approval, serious cardiovascular adverse events (CVAEs) may not be apparent in initial trials. Methods: A retrospective analysis of CVAEs linked to five World Health Organization (WHO) recommended COVID-19 therapies was conducted using the WHO VigiBase database from March 2020 to July 2023. Adjusted reporting odds ratios (aROR) with 95% confidence intervals (CI) were calculated to assess CVAE risks. Results: A total of 276,631 AEs were reported to be associated with COVID-19, of which 13,091 were classified as cardiovascular events. Remdesivir was associated with significantly increased odds of CVAEs, particularly bradycardia (aROR 2.4, 95% CI 2.28-2.52). In contrast, nirmatrelvir/ritonavir and molnupiravir showed reduced CVAEs odds. Among immunomodulators, baricitinib was associated with increased
Publisher's Note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Aljuhani, Sulaiman, Korayem, Altebainawi, Alsohimi et al., The association between tocilizumab therapy and the development of thrombosis in critically ill patients with COVID-19: a multicenter, cohort study, Sci Rep
Alsowaida, Shehadeh, Kalligeros, Mylonakis, Incidence and potential risk factors for remdesivir-associated bradycardia in hospitalized patients with COVID-19: a retrospective cohort study, Front Pharmacol
Alsulaim, Alhassan, Khalil, Almutlaq, Tocilizumab effect on lipid profile in correlation to cardiovascular events: a retrospective cohort study, Int J Rheumatol
Apostolova, Blas-Garcia, Esplugues, Mitochondrial interference by anti-HIV drugs: mechanisms beyond Pol-gamma inhibition, Trends Pharmacol Sci
Bate, Lindquist, Edwards, Olsson, Orre et al., A Bayesian neural network method for adverse drug reaction signal generation, Eur J Clin Pharmacol
Bernal, Da Silva, Musungaie, Kovalchuk, Gonzalez et al., Molnupiravir for oral treatment of COVID-19 in nonhospitalized patients, N Engl J Med
Chenchula, Karunakaran, Sharma, Chavan, Current evidence on efficacy of COVID-19 booster dose vaccination against the Omicron variant: a systematic review, J Med Virol
Choi, Shin, Park, Park, Lee, Antiviral activity and safety of remdesivir against SARS-CoV-2 infection in human pluripotent stem cell-derived cardiomyocytes, Antivir Res
Demarco, Turnipseed, Clarke, Qadeer, Possible nirmatrelvir/ritonavir-induced bradycardia in a patient with asymptomatic COVID-19, SAGE Open Med Case Rep
Focosi, Franchini, Maggi, Shoham, COVID-19 therapeutics, Clin Microbiol Rev
Ganipisetti, Bollimunta, Maringanti, Paxlovid-induced symptomatic bradycardia and syncope, Cureus
Gu, Han, Wang, Zhang, The impacts of nirmatrelvir-ritonavir on myocardial injury and long-term cardiovascular outcomes in hospitalized patients with COVID-19 amid the Omicron wave of the pandemic, Cardiovasc Drugs Ther, doi:10.1007/s10557-024-07570-4
Hilser, Spencer, Afshari, Gilliland, Hu, COVID-19 is a coronary artery disease risk equivalent and exhibits a genetic interaction with ABO blood type, Arterioscler Thromb Vasc Biol
Jung, Kim, Li, Lee, Koyanagi et al., Cardiovascular events and safety outcomes associated with remdesivir using a World Health Organization international pharmacovigilance database, Clin Transl Sci
Karampitsakos, Papaioannou, Tsiri, Katsaras, Katsimpris et al., Tocilizumab versus baricitinib in hospitalized patients with severe COVID-19: an open label, randomized controlled trial, Clin Microbiol Infect
Khetran, Mustafa, Mutations of SARS-CoV-2 structural proteins in the Alpha, Beta, Gamma, and Delta variants: bioinformatics analysis, JMIR Bioinform Biotechnol
Knight, Walker, Ip, Cooper, Bolton et al., Association of COVID-19 with major arterial and venous thrombotic diseases: a population-wide cohort study of 48 million adults in England and Wales, Circulation
Kotyla, Engelmann, Giemza-Stokłosa, Wnuk, Islam, Thromboembolic adverse drug reactions in Janus kinase (JAK) inhibitors: does the inhibitor specificity play a role?, Int J Mol Sci, doi:10.3390/ijms22052449
Kragstrup, Glintborg, Svensson, Mcmaster, Robinson et al., Waiting for JAK inhibitor safety data, RMD Open, doi:10.1136/rmdopen-2022-002236
Lei, Chen, Wu, Duan, Men, Small molecules in the treatment of COVID-19, Signal Transduct Target Ther
Liang, Ma, Wang, Zheng, Su et al., Adverse events associated with molnupiravir: a real-world disproportionality analysis in the Food and Drug Administration Adverse Event Reporting System, Front Pharmacol
Lindquist, Vigibase, the WHO global ICSR database system: basic facts, Drug Inf J DIJ/Drug Inf Assoc
Marconi, Ramanan, De Bono, Kartman, Krishnan et al., Efficacy and safety of baricitinib for the treatment of hospitalised adults with COVID-19 (COV-BARRIER): a randomised, double-blind, parallel-group, placebocontrolled phase 3 trial, Lancet Respir Med
Mok, Kwok, Li, Ling, Lai et al., SARS-CoV-2 variants divergently infect and damage cardiomyocytes in vitro and in vivo, Cell Biosci
Molander, Bower, Frisell, Delcoigne, Giuseppe et al., Venous thromboembolism with JAK inhibitors and other immunemodulatory drugs: a Swedish comparative safety study among patients with rheumatoid arthritis, Ann Rheum Dis
Nabati, Parsaee, Potential cardiotoxic effects of remdesivir on cardiovascular system: a literature review, Cardiovasc Toxicol
Noren, Hopstadius, Bate, Shrinkage observed-to-expected ratios for robust and transparent large-scale pattern discovery, Stat Methods Med Res
Padasas, Espano, Kim, Song, Lee et al., COVID-19 therapeutics: an update on effective treatments against infection with SARS-CoV-2 variants, Immune Netw
Pruijssers, George, Schafer, Leist, Gralinksi et al., Remdesivir inhibits SARS-CoV-2 in human lung cells and chimeric SARS-CoV expressing the SARS-CoV-2 RNA polymerase in mice, Cell Rep
Raschi, Salvo, Khouri, Conceiving, conducting, reporting, interpreting, and publishing disproportionality analyses: a call to action, Br J Clin Pharmacol
Rubin, Baricitinib is first approved COVID-19 immunomodulatory treatment, JAMA
Rubin, Chan-Tack, Farley, Sherwat, FDA approval of remdesivir-a step in the right direction, N Engl J Med
Santi Laurini, Montanaro, Motola, Safety profile of molnupiravir in the treatment of COVID-19: a descriptive study based on FAERS data, J Clin Med
Shafran, Alasti, Smolen, Aletaha, Implication of baseline levels and early changes of C-reactive protein for subsequent clinical outcomes of patients with rheumatoid arthritis treated with tocilizumab, Ann Rheum Dis
Sharma, Garcia, Wang, Plummer, Morizono et al., Human iPSCderived cardiomyocytes are susceptible to SARS-CoV-2 infection, Cell Rep Med
Shuai, Chan, Hu, Chai, Yuen et al., Attenuated replication and pathogenicity of SARS-CoV-2 B.1.1.529 Omicron, Nature
Sun, Wang, Ma, Wei, Peng et al., Efficacy and safety of baricitinib for the treatment of hospitalized adults with COVID-19: a systematic review and meta-analysis, Eur J Med Res
Terzic, Basilua, Billard, De Gastines, Belhadi et al., Cardiac adverse events and remdesivir in hospitalized patients with COVID-19: a post hoc safety analysis of the randomized DisCoVeRy Trial, Clin Infect Dis
Vj, Illescas-Montes, Puerta-Puerta, Ruiz, Melguizo-Rodriguez, SARS-CoV-2 infection: the role of cytokines in COVID-19 disease, Cytokine Growth Factor Rev
Wang, Jiang, Li, Liu, Standardizing adverse drug event reporting data, J Biomed Semant
Wang, Wang, Huang, Hsieh, Ibarburu et al., Paxlovid use is associated with lower risk of cardiovascular diseases in COVID-19 patients with autoimmune rheumatic diseases: a retrospective cohort study, BMC Med
Zou, Jing, Cardiovascular adverse events associated with monoclonal antibody products in patients with COVID-19, Pharmaceuticals, doi:10.3390/ph15121472
DOI record:
{
"DOI": "10.1007/s40121-025-01225-z",
"ISSN": [
"2193-8229",
"2193-6382"
],
"URL": "http://dx.doi.org/10.1007/s40121-025-01225-z",
"alternative-id": [
"1225"
],
"assertion": [
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Received",
"name": "received",
"order": 1,
"value": "23 July 2025"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Accepted",
"name": "accepted",
"order": 2,
"value": "18 August 2025"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "First Online",
"name": "first_online",
"order": 3,
"value": "11 September 2025"
},
{
"group": {
"label": "Declarations",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 1
},
{
"group": {
"label": "Conflict of interest",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 2,
"value": "Hoi Ki Cheng is an employee of Pfizer Inc, but the latter did not play any role in the design and execution of this study. All other authors (Angel Lai, Maxwell Kwok, Bryan P Yan, Ellen N Poon) have no conflicts of interest relevant to the content of this manuscript."
},
{
"group": {
"label": "Ethical approval",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 3,
"value": "This article is based on available data in VigiBase (), and the data is anonymous and is available upon request from said database. This manuscript does not contain any new studies with human participants or animals performed by any of the authors. The study was approved by the ethics committee of The Chinese University of Hong Kong (CUHK) and New Territories East Cluster (NTEC) (Ref. No. 2023.186). Since only anonymous data was collected, informed consent was waived."
}
],
"author": [
{
"ORCID": "https://orcid.org/0009-0000-0409-5930",
"affiliation": [],
"authenticated-orcid": false,
"family": "Cheng",
"given": "Hoi K.",
"sequence": "first"
},
{
"ORCID": "https://orcid.org/0000-0001-5262-661X",
"affiliation": [],
"authenticated-orcid": false,
"family": "Lai",
"given": "Angel",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0001-5920-9341",
"affiliation": [],
"authenticated-orcid": false,
"family": "Kwok",
"given": "Maxwell",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0003-0430-5752",
"affiliation": [],
"authenticated-orcid": false,
"family": "Yan",
"given": "Bryan P.",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0002-9280-3681",
"affiliation": [],
"authenticated-orcid": false,
"family": "Poon",
"given": "Ellen Ngar-Yun",
"sequence": "additional"
}
],
"container-title": "Infectious Diseases and Therapy",
"container-title-short": "Infect Dis Ther",
"content-domain": {
"crossmark-restriction": false,
"domain": [
"link.springer.com"
]
},
"created": {
"date-parts": [
[
2025,
9,
11
]
],
"date-time": "2025-09-11T02:33:26Z",
"timestamp": 1757558006000
},
"deposited": {
"date-parts": [
[
2025,
9,
29
]
],
"date-time": "2025-09-29T09:27:18Z",
"timestamp": 1759138038000
},
"funder": [
{
"DOI": "10.13039/501100005847",
"award": [
"22210062"
],
"doi-asserted-by": "crossref",
"id": [
{
"asserted-by": "crossref",
"id": "10.13039/501100005847",
"id-type": "DOI"
}
],
"name": "Health and Medical Research Fund"
}
],
"indexed": {
"date-parts": [
[
2025,
9,
29
]
],
"date-time": "2025-09-29T09:40:18Z",
"timestamp": 1759138818760,
"version": "3.44.0"
},
"is-referenced-by-count": 1,
"issue": "10",
"issued": {
"date-parts": [
[
2025,
9,
11
]
]
},
"journal-issue": {
"issue": "10",
"published-print": {
"date-parts": [
[
2025,
10
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by-nc/4.0",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2025,
9,
11
]
],
"date-time": "2025-09-11T00:00:00Z",
"timestamp": 1757548800000
}
},
{
"URL": "https://creativecommons.org/licenses/by-nc/4.0",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2025,
9,
11
]
],
"date-time": "2025-09-11T00:00:00Z",
"timestamp": 1757548800000
}
}
],
"link": [
{
"URL": "https://link.springer.com/content/pdf/10.1007/s40121-025-01225-z.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://link.springer.com/article/10.1007/s40121-025-01225-z/fulltext.html",
"content-type": "text/html",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://link.springer.com/content/pdf/10.1007/s40121-025-01225-z.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "297",
"original-title": [],
"page": "2445-2463",
"prefix": "10.1007",
"published": {
"date-parts": [
[
2025,
9,
11
]
]
},
"published-online": {
"date-parts": [
[
2025,
9,
11
]
]
},
"published-print": {
"date-parts": [
[
2025,
10
]
]
},
"publisher": "Springer Science and Business Media LLC",
"reference": [
{
"DOI": "10.1128/cmr.00119-23",
"author": "D Focosi",
"doi-asserted-by": "publisher",
"first-page": "e0011923",
"issue": "2",
"journal-title": "Clin Microbiol Rev",
"key": "1225_CR1",
"unstructured": "Focosi D, Franchini M, Maggi F, Shoham S. COVID-19 therapeutics. Clin Microbiol Rev. 2024;37(2):e0011923.",
"volume": "37",
"year": "2024"
},
{
"DOI": "10.1038/s41392-022-01249-8",
"author": "S Lei",
"doi-asserted-by": "publisher",
"first-page": "387",
"issue": "1",
"journal-title": "Signal Transduct Target Ther",
"key": "1225_CR2",
"unstructured": "Lei S, Chen X, Wu J, Duan X, Men K. Small molecules in the treatment of COVID-19. Signal Transduct Target Ther. 2022;7(1):387.",
"volume": "7",
"year": "2022"
},
{
"DOI": "10.1016/j.celrep.2020.107940",
"author": "AJ Pruijssers",
"doi-asserted-by": "publisher",
"issue": "3",
"journal-title": "Cell Rep",
"key": "1225_CR3",
"unstructured": "Pruijssers AJ, George AS, Schafer A, Leist SR, Gralinksi LE, Dinnon KH 3rd, et al. Remdesivir inhibits SARS-CoV-2 in human lung cells and chimeric SARS-CoV expressing the SARS-CoV-2 RNA polymerase in mice. Cell Rep. 2020;32(3): 107940.",
"volume": "32",
"year": "2020"
},
{
"DOI": "10.1056/NEJMoa2116044",
"author": "A Jayk Bernal",
"doi-asserted-by": "publisher",
"first-page": "509",
"issue": "6",
"journal-title": "N Engl J Med",
"key": "1225_CR4",
"unstructured": "Jayk Bernal A, Gomes da Silva MM, Musungaie DB, Kovalchuk E, Gonzalez A, Delos Reyes V, et al. Molnupiravir for oral treatment of COVID-19 in nonhospitalized patients. N Engl J Med. 2022;386(6):509–20.",
"volume": "386",
"year": "2022"
},
{
"DOI": "10.1016/j.cytogfr.2020.06.001",
"author": "VJ Costela-Ruiz",
"doi-asserted-by": "publisher",
"first-page": "62",
"journal-title": "Cytokine Growth Factor Rev",
"key": "1225_CR5",
"unstructured": "Costela-Ruiz VJ, Illescas-Montes R, Puerta-Puerta JM, Ruiz C, Melguizo-Rodriguez L. SARS-CoV-2 infection: the role of cytokines in COVID-19 disease. Cytokine Growth Factor Rev. 2020;54:62–75.",
"volume": "54",
"year": "2020"
},
{
"author": "R Rubin",
"first-page": "2281",
"issue": "23",
"journal-title": "JAMA",
"key": "1225_CR6",
"unstructured": "Rubin R. Baricitinib is first approved COVID-19 immunomodulatory treatment. JAMA. 2022;327(23):2281.",
"volume": "327",
"year": "2022"
},
{
"DOI": "10.1136/annrheumdis-2019-215987",
"author": "IH Shafran",
"doi-asserted-by": "publisher",
"first-page": "874",
"issue": "7",
"journal-title": "Ann Rheum Dis",
"key": "1225_CR7",
"unstructured": "Shafran IH, Alasti F, Smolen JS, Aletaha D. Implication of baseline levels and early changes of C-reactive protein for subsequent clinical outcomes of patients with rheumatoid arthritis treated with tocilizumab. Ann Rheum Dis. 2020;79(7):874–82.",
"volume": "79",
"year": "2020"
},
{
"DOI": "10.1016/j.tips.2011.07.007",
"author": "N Apostolova",
"doi-asserted-by": "publisher",
"first-page": "715",
"issue": "12",
"journal-title": "Trends Pharmacol Sci",
"key": "1225_CR8",
"unstructured": "Apostolova N, Blas-Garcia A, Esplugues JV. Mitochondrial interference by anti-HIV drugs: mechanisms beyond Pol-gamma inhibition. Trends Pharmacol Sci. 2011;32(12):715–25.",
"volume": "32",
"year": "2011"
},
{
"DOI": "10.1016/j.xcrm.2020.100052",
"author": "A Sharma",
"doi-asserted-by": "publisher",
"issue": "4",
"journal-title": "Cell Rep Med",
"key": "1225_CR9",
"unstructured": "Sharma A, Garcia G Jr., Wang Y, Plummer JT, Morizono K, Arumugaswami V, et al. Human iPSC-derived cardiomyocytes are susceptible to SARS-CoV-2 infection. Cell Rep Med. 2020;1(4): 100052.",
"volume": "1",
"year": "2020"
},
{
"DOI": "10.1136/ard-2022-223050",
"author": "V Molander",
"doi-asserted-by": "publisher",
"first-page": "189",
"issue": "2",
"journal-title": "Ann Rheum Dis",
"key": "1225_CR10",
"unstructured": "Molander V, Bower H, Frisell T, Delcoigne B, Di Giuseppe D, Askling J, et al. Venous thromboembolism with JAK inhibitors and other immune-modulatory drugs: a Swedish comparative safety study among patients with rheumatoid arthritis. Ann Rheum Dis. 2023;82(2):189–97.",
"volume": "82",
"year": "2023"
},
{
"DOI": "10.3390/ph15121472",
"author": "J Zou",
"doi-asserted-by": "publisher",
"journal-title": "Pharmaceuticals (Basel)",
"key": "1225_CR11",
"unstructured": "Zou J, Jing F. Cardiovascular adverse events associated with monoclonal antibody products in patients with COVID-19. Pharmaceuticals (Basel). 2022. https://doi.org/10.3390/ph15121472.",
"year": "2022"
},
{
"DOI": "10.1186/2041-1480-5-36",
"author": "L Wang",
"doi-asserted-by": "publisher",
"journal-title": "J Biomed Semant",
"key": "1225_CR12",
"unstructured": "Wang L, Jiang G, Li D, Liu H. Standardizing adverse drug event reporting data. J Biomed Semant. 2014;5: 36.",
"volume": "5",
"year": "2014"
},
{
"DOI": "10.1177/009286150804200501",
"author": "M Lindquist",
"doi-asserted-by": "publisher",
"first-page": "409",
"issue": "5",
"journal-title": "Drug Inf J DIJ/Drug Inf Assoc",
"key": "1225_CR13",
"unstructured": "Lindquist M. Vigibase, the WHO global ICSR database system: basic facts. Drug Inf J DIJ/Drug Inf Assoc. 2008;42(5):409–19.",
"volume": "42",
"year": "2008"
},
{
"key": "1225_CR14",
"unstructured": "Uppsala Monitoring Centre. VigiBase, WHO’s global database 2024. https://who-umc.org/vigibase/vigibase-who-s-global-database/."
},
{
"DOI": "10.1002/jmv.27697",
"author": "S Chenchula",
"doi-asserted-by": "publisher",
"first-page": "2969",
"issue": "7",
"journal-title": "J Med Virol",
"key": "1225_CR15",
"unstructured": "Chenchula S, Karunakaran P, Sharma S, Chavan M. Current evidence on efficacy of COVID-19 booster dose vaccination against the Omicron variant: a systematic review. J Med Virol. 2022;94(7):2969–76.",
"volume": "94",
"year": "2022"
},
{
"key": "1225_CR16",
"unstructured": "Uppsala Monitoring Centre. Guideline for using VigiBase data in studies. 2021."
},
{
"DOI": "10.1111/bcp.15269",
"author": "E Raschi",
"doi-asserted-by": "publisher",
"first-page": "3535",
"issue": "7",
"journal-title": "Br J Clin Pharmacol",
"key": "1225_CR17",
"unstructured": "Raschi E, Salvo F, Khouri C. Conceiving, conducting, reporting, interpreting, and publishing disproportionality analyses: a call to action. Br J Clin Pharmacol. 2022;88(7):3535–6.",
"volume": "88",
"year": "2022"
},
{
"DOI": "10.1177/0962280211403604",
"author": "GN Noren",
"doi-asserted-by": "publisher",
"first-page": "57",
"issue": "1",
"journal-title": "Stat Methods Med Res",
"key": "1225_CR18",
"unstructured": "Noren GN, Hopstadius J, Bate A. Shrinkage observed-to-expected ratios for robust and transparent large-scale pattern discovery. Stat Methods Med Res. 2013;22(1):57–69.",
"volume": "22",
"year": "2013"
},
{
"DOI": "10.1007/s002280050466",
"author": "A Bate",
"doi-asserted-by": "publisher",
"first-page": "315",
"issue": "4",
"journal-title": "Eur J Clin Pharmacol",
"key": "1225_CR19",
"unstructured": "Bate A, Lindquist M, Edwards IR, Olsson S, Orre R, Lansner A, et al. A Bayesian neural network method for adverse drug reaction signal generation. Eur J Clin Pharmacol. 1998;54(4):315–21.",
"volume": "54",
"year": "1998"
},
{
"DOI": "10.2196/43906",
"author": "SR Khetran",
"doi-asserted-by": "publisher",
"journal-title": "JMIR Bioinform Biotechnol",
"key": "1225_CR20",
"unstructured": "Khetran SR, Mustafa R. Mutations of SARS-CoV-2 structural proteins in the Alpha, Beta, Gamma, and Delta variants: bioinformatics analysis. JMIR Bioinform Biotechnol. 2023;4: e43906.",
"volume": "4",
"year": "2023"
},
{
"key": "1225_CR21",
"unstructured": "U.S. Food and Drug Administration. Coronavirus (COVID-19) Update: FDA Issues Emergency Use Authorization for Potential COVID-19 Treatment May 01, 2020. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-issues-emergency-use-authorization-potential-covid-19-treatment."
},
{
"DOI": "10.1056/NEJMp2032369",
"author": "D Rubin",
"doi-asserted-by": "publisher",
"first-page": "2598",
"issue": "27",
"journal-title": "N Engl J Med",
"key": "1225_CR22",
"unstructured": "Rubin D, Chan-Tack K, Farley J, Sherwat A. FDA approval of remdesivir—a step in the right direction. N Engl J Med. 2020;383(27):2598–600.",
"volume": "383",
"year": "2020"
},
{
"key": "1225_CR23",
"unstructured": "U.S. Food and Drug Administration. Coronavirus (COVID-19) Update: FDA Authorizes Drug Combination for Treatment of COVID-19 November 19. 2020. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-drug-combination-treatment-covid-19."
},
{
"key": "1225_CR24",
"unstructured": "U.S. Food and Drug Administration. Coronavirus (COVID-19) Update: FDA Authorizes Drug for Treatment of COVID-19 June 24, 2021. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-drug-treatment-covid-19."
},
{
"DOI": "10.4110/in.2023.23.e13",
"author": "BT Padasas",
"doi-asserted-by": "publisher",
"issue": "2",
"journal-title": "Immune Netw",
"key": "1225_CR25",
"unstructured": "Padasas BT, Espano E, Kim SH, Song Y, Lee CK, Kim JK. COVID-19 therapeutics: an update on effective treatments against infection with SARS-CoV-2 variants. Immune Netw. 2023;23(2): e13.",
"volume": "23",
"year": "2023"
},
{
"DOI": "10.1007/s10557-024-07570-4",
"author": "J Gu",
"doi-asserted-by": "publisher",
"journal-title": "Cardiovasc Drugs Ther",
"key": "1225_CR26",
"unstructured": "Gu J, Han ZH, Wang CQ, Zhang JF. The impacts of nirmatrelvir-ritonavir on myocardial injury and long-term cardiovascular outcomes in hospitalized patients with COVID-19 amid the Omicron wave of the pandemic. Cardiovasc Drugs Ther. 2024. https://doi.org/10.1007/s10557-024-07570-4.",
"year": "2024"
},
{
"DOI": "10.1186/s12916-024-03331-0",
"author": "W Wang",
"doi-asserted-by": "publisher",
"first-page": "117",
"issue": "1",
"journal-title": "BMC Med",
"key": "1225_CR27",
"unstructured": "Wang W, Wang YH, Huang CH, Hsieh TH, Ibarburu GH, Wei JC. Paxlovid use is associated with lower risk of cardiovascular diseases in COVID-19 patients with autoimmune rheumatic diseases: a retrospective cohort study. BMC Med. 2024;22(1):117.",
"volume": "22",
"year": "2024"
},
{
"DOI": "10.1177/2050313X231168304",
"author": "E DeMarco",
"doi-asserted-by": "publisher",
"journal-title": "SAGE Open Med Case Rep",
"key": "1225_CR28",
"unstructured": "DeMarco E, Turnipseed M, Clarke B, Qadeer F. Possible nirmatrelvir/ritonavir-induced bradycardia in a patient with asymptomatic COVID-19. SAGE Open Med Case Rep. 2023;11: 2050313X231168304.",
"volume": "11",
"year": "2023"
},
{
"author": "VM Ganipisetti",
"first-page": "e33831",
"issue": "1",
"journal-title": "Cureus",
"key": "1225_CR29",
"unstructured": "Ganipisetti VM, Bollimunta P, Maringanti S. Paxlovid-induced symptomatic bradycardia and syncope. Cureus. 2023;15(1):e33831.",
"volume": "15",
"year": "2023"
},
{
"DOI": "10.3389/fphar.2023.1253799",
"author": "Y Liang",
"doi-asserted-by": "publisher",
"first-page": "1253799",
"journal-title": "Front Pharmacol",
"key": "1225_CR30",
"unstructured": "Liang Y, Ma L, Wang Y, Zheng J, Su L, Lyu J. Adverse events associated with molnupiravir: a real-world disproportionality analysis in the Food and Drug Administration Adverse Event Reporting System. Front Pharmacol. 2023;14:1253799.",
"volume": "14",
"year": "2023"
},
{
"DOI": "10.3390/jcm12010034",
"author": "G Santi Laurini",
"doi-asserted-by": "publisher",
"first-page": "34",
"issue": "1",
"journal-title": "J Clin Med",
"key": "1225_CR31",
"unstructured": "Santi Laurini G, Montanaro N, Motola D. Safety profile of molnupiravir in the treatment of COVID-19: a descriptive study based on FAERS data. J Clin Med. 2022;12(1):34.",
"volume": "12",
"year": "2022"
},
{
"key": "1225_CR32",
"unstructured": "World Health Organization. Therapeutics and COVID-19: living guideline. Geneva; 2023 10 November. Report No.: WHO/2019-nCoV/clinical/2023.2."
},
{
"key": "1225_CR33",
"unstructured": "U.S. Food and Drug Administration. Fact Sheet for Patients and Caregivers: Emergency Use Authorization (EUA) of LAGEVRIO™ (molnupiravir) capsules for Coronavirus Disease 2019 (COVID-19) 2024. https://www.fda.gov/media/155055/download. Accessed June 2024."
},
{
"DOI": "10.1093/cid/ciae170",
"author": "V Terzic",
"doi-asserted-by": "publisher",
"first-page": "382",
"issue": "2",
"journal-title": "Clin Infect Dis",
"key": "1225_CR34",
"unstructured": "Terzic V, Miantezila Basilua J, Billard N, de Gastines L, Belhadi D, Fougerou-Leurent C, et al. Cardiac adverse events and remdesivir in hospitalized patients with COVID-19: a post hoc safety analysis of the randomized DisCoVeRy Trial. Clin Infect Dis. 2024;79(2):382–91.",
"volume": "79",
"year": "2024"
},
{
"DOI": "10.3389/fphar.2023.1106044",
"author": "YS Alsowaida",
"doi-asserted-by": "publisher",
"first-page": "1106044",
"journal-title": "Front Pharmacol",
"key": "1225_CR35",
"unstructured": "Alsowaida YS, Shehadeh F, Kalligeros M, Mylonakis E. Incidence and potential risk factors for remdesivir-associated bradycardia in hospitalized patients with COVID-19: a retrospective cohort study. Front Pharmacol. 2023;14:1106044.",
"volume": "14",
"year": "2023"
},
{
"DOI": "10.1111/cts.13168",
"author": "SY Jung",
"doi-asserted-by": "publisher",
"first-page": "501",
"issue": "2",
"journal-title": "Clin Transl Sci",
"key": "1225_CR36",
"unstructured": "Jung SY, Kim MS, Li H, Lee KH, Koyanagi A, Solmi M, et al. Cardiovascular events and safety outcomes associated with remdesivir using a World Health Organization international pharmacovigilance database. Clin Transl Sci. 2022;15(2):501–13.",
"volume": "15",
"year": "2022"
},
{
"DOI": "10.1007/s12012-021-09703-9",
"author": "M Nabati",
"doi-asserted-by": "publisher",
"first-page": "268",
"issue": "3",
"journal-title": "Cardiovasc Toxicol",
"key": "1225_CR37",
"unstructured": "Nabati M, Parsaee H. Potential cardiotoxic effects of remdesivir on cardiovascular system: a literature review. Cardiovasc Toxicol. 2022;22(3):268–72.",
"volume": "22",
"year": "2022"
},
{
"DOI": "10.1016/j.antiviral.2020.104955",
"author": "SW Choi",
"doi-asserted-by": "publisher",
"journal-title": "Antivir Res",
"key": "1225_CR38",
"unstructured": "Choi SW, Shin JS, Park SJ, Jung E, Park YG, Lee J, et al. Antiviral activity and safety of remdesivir against SARS-CoV-2 infection in human pluripotent stem cell-derived cardiomyocytes. Antivir Res. 2020;184: 104955.",
"volume": "184",
"year": "2020"
},
{
"DOI": "10.1186/s13578-024-01280-y",
"author": "BW Mok",
"doi-asserted-by": "publisher",
"first-page": "101",
"issue": "1",
"journal-title": "Cell Biosci",
"key": "1225_CR39",
"unstructured": "Mok BW, Kwok M, Li HS, Ling L, Lai A, Yan B, et al. SARS-CoV-2 variants divergently infect and damage cardiomyocytes in vitro and in vivo. Cell Biosci. 2024;14(1):101.",
"volume": "14",
"year": "2024"
},
{
"DOI": "10.1016/S2213-2600(21)00331-3",
"author": "VC Marconi",
"doi-asserted-by": "publisher",
"first-page": "1407",
"issue": "12",
"journal-title": "Lancet Respir Med",
"key": "1225_CR40",
"unstructured": "Marconi VC, Ramanan AV, de Bono S, Kartman CE, Krishnan V, Liao R, et al. Efficacy and safety of baricitinib for the treatment of hospitalised adults with COVID-19 (COV-BARRIER): a randomised, double-blind, parallel-group, placebo-controlled phase 3 trial. Lancet Respir Med. 2021;9(12):1407–18.",
"volume": "9",
"year": "2021"
},
{
"DOI": "10.1186/s40001-023-01403-0",
"author": "J Sun",
"doi-asserted-by": "publisher",
"issue": "1",
"journal-title": "Eur J Med Res",
"key": "1225_CR41",
"unstructured": "Sun J, Wang S, Ma X, Wei Q, Peng Y, Bai Y, et al. Efficacy and safety of baricitinib for the treatment of hospitalized adults with COVID-19: a systematic review and meta-analysis. Eur J Med Res. 2023;28(1): 536.",
"volume": "28",
"year": "2023"
},
{
"DOI": "10.1155/2021/5535486",
"author": "T Alsulaim",
"doi-asserted-by": "publisher",
"first-page": "5535486",
"journal-title": "Int J Rheumatol",
"key": "1225_CR42",
"unstructured": "Alsulaim T, Alhassan N, Khalil H, Almutlaq A. Tocilizumab effect on lipid profile in correlation to cardiovascular events: a retrospective cohort study. Int J Rheumatol. 2021;2021:5535486.",
"volume": "2021",
"year": "2021"
},
{
"DOI": "10.1038/s41598-024-53087-z",
"author": "O Aljuhani",
"doi-asserted-by": "publisher",
"issue": "1",
"journal-title": "Sci Rep",
"key": "1225_CR43",
"unstructured": "Aljuhani O, Al Sulaiman K, Korayem GB, Altebainawi AF, Alsohimi S, Alqahtani R, et al. The association between tocilizumab therapy and the development of thrombosis in critically ill patients with COVID-19: a multicenter, cohort study. Sci Rep. 2024;14(1): 3037.",
"volume": "14",
"year": "2024"
},
{
"DOI": "10.58347/tml.2023.1667d",
"doi-asserted-by": "crossref",
"key": "1225_CR44",
"unstructured": "COVID-19 update: Tocilizumab (Actemra) FDA-approved for treatment of COVID-19. Med Lett Drugs Ther. 2023;65(1667):e9."
},
{
"DOI": "10.1136/rmdopen-2022-002236",
"author": "TW Kragstrup",
"doi-asserted-by": "publisher",
"journal-title": "RMD Open",
"key": "1225_CR45",
"unstructured": "Kragstrup TW, Glintborg B, Svensson AL, McMaster C, Robinson PC, Deleuran B, et al. Waiting for JAK inhibitor safety data. RMD Open. 2022. https://doi.org/10.1136/rmdopen-2022-002236.",
"year": "2022"
},
{
"DOI": "10.3390/ijms22052449",
"author": "PJ Kotyla",
"doi-asserted-by": "publisher",
"journal-title": "Int J Mol Sci",
"key": "1225_CR46",
"unstructured": "Kotyla PJ, Engelmann M, Giemza-Stokłosa J, Wnuk B, Islam MA. Thromboembolic adverse drug reactions in Janus kinase (JAK) inhibitors: does the inhibitor specificity play a role? Int J Mol Sci. 2021. https://doi.org/10.3390/ijms22052449.",
"year": "2021"
},
{
"DOI": "10.1161/CIRCULATIONAHA.122.060785",
"author": "R Knight",
"doi-asserted-by": "publisher",
"first-page": "892",
"issue": "12",
"journal-title": "Circulation",
"key": "1225_CR47",
"unstructured": "Knight R, Walker V, Ip S, Cooper JA, Bolton T, Keene S, et al. Association of COVID-19 with major arterial and venous thrombotic diseases: a population-wide cohort study of 48 million adults in England and Wales. Circulation. 2022;146(12):892–906.",
"volume": "146",
"year": "2022"
},
{
"DOI": "10.1038/s41586-022-04442-5",
"author": "H Shuai",
"doi-asserted-by": "publisher",
"first-page": "693",
"issue": "7902",
"journal-title": "Nature",
"key": "1225_CR48",
"unstructured": "Shuai H, Chan JF, Hu B, Chai Y, Yuen TT, Yin F, et al. Attenuated replication and pathogenicity of SARS-CoV-2 B.1.1.529 Omicron. Nature. 2022;603(7902):693–9.",
"volume": "603",
"year": "2022"
},
{
"DOI": "10.1161/ATVBAHA.124.321001",
"author": "JR Hilser",
"doi-asserted-by": "publisher",
"first-page": "2321",
"issue": "11",
"journal-title": "Arterioscler Thromb Vasc Biol",
"key": "1225_CR49",
"unstructured": "Hilser JR, Spencer NJ, Afshari K, Gilliland FD, Hu H, Deb A, et al. COVID-19 is a coronary artery disease risk equivalent and exhibits a genetic interaction with ABO blood type. Arterioscler Thromb Vasc Biol. 2024;44(11):2321–33.",
"volume": "44",
"year": "2024"
},
{
"DOI": "10.1016/j.cmi.2022.10.015",
"author": "T Karampitsakos",
"doi-asserted-by": "publisher",
"first-page": "372",
"issue": "3",
"journal-title": "Clin Microbiol Infect",
"key": "1225_CR50",
"unstructured": "Karampitsakos T, Papaioannou O, Tsiri P, Katsaras M, Katsimpris A, Kalogeropoulos AP, et al. Tocilizumab versus baricitinib in hospitalized patients with severe COVID-19: an open label, randomized controlled trial. Clin Microbiol Infect. 2023;29(3):372–8.",
"volume": "29",
"year": "2023"
}
],
"reference-count": 50,
"references-count": 50,
"relation": {},
"resource": {
"primary": {
"URL": "https://link.springer.com/10.1007/s40121-025-01225-z"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Cardiovascular Safety of COVID-19 Treatments: A Disproportionality Analysis of Adverse Event Reports from the WHO VigiBase",
"type": "journal-article",
"update-policy": "https://doi.org/10.1007/springer_crossmark_policy",
"volume": "14"
}